Prev Page--Structure || Next Page--Well Records
Ground Water
Source and Principles of Occurrence
The source and principles of occurrence of ground water as related to Brown County are discussed on the following pages. The principles governing the occurrence of ground water have been discussed by many authors (Meinzer, 1923a; Moore, et al., 1940) and the reader is referred to their reports for a more detailed discussion of the subject.
Water in the pores or interstices of rocks in the zone that is completely saturated is called ground water. In Brown County ground water is derived from precipitation in the form of rain or snow that falls on the county or in nearby areas. Part of the precipitation leaves as runoff discharged by streams, part evaporates, and part is transpired by vegetation. The part that escapes runoff, evaporation, or transpiration moves slowly downward through the soil and the underlying strata until it reaches the zone of saturation. After reaching the ground-water body, the water percolates through the rocks in a direction determined by the geology, topography, and geologic structure, until it is discharged by wells and springs, or by evaporation and transpiration in areas where the water table is relatively near the surface, or it is discharged directly into streams or other bodies of water.
The amount of water that can be stored in a water-bearing formation is dependent upon the porosity of the formation. Porosity is expressed as the ratio of the volume of interstices in the material to the total volume of the material. Saturated rocks of high porosity do not necessarily yield large quantities of water to wells; one rock may readily yield most of the water contained in its pores, but another having equal porosity but smaller pores may retain most of its contained water due to capillary attraction.
The quantity of water a water-bearing material will yield and the rate at which water will move through it are governed by its physical and hydrologic properties. Sediments are seldom homogeneous; their physical and hydrologic properties range widely, being governed by the size, shape, number, and degree of interconnection of the voids in the material. A water-bearing rock may have high porosity in relatively large voids, but, unless the voids are interconnected, little water can move through it, resulting in low permeability and transmissibility. Under such conditions a satisfactory well cannot be obtained.
Permeability may be defined as the capacity of rock to transmit water. The field coefficient of permeability of an aquifer may be expressed as the rate of flow of water at the prevailing temperature, in gallons a day, through a cross-sectional area having a thickness of 1 foot and a width of 1 mile for each foot per mile of hydraulic gradient. The coefficient of transmissibility is the field coefficient of permeability multiplied by the saturated thickness, in feet, of the aquifer.
The water table is the upper surface of the zone of saturation except where that surface is formed by an impermeable body. If the upper surface of the zone of saturation is formed by an impermeable body, no water table exists. The water level in a well drilled into saturated material under water-table conditions will stand in the well at the level at which it was first reached. When water is confined in a bed under pressure by an impermeable bed, artesian conditions exist. The water in a well drilled into an artesian aquifer will rise to a level higher than the level at which it was reached; if the pressure is great enough to lift the water above the land surface, the well will flow. In Brown County much of the water contained in the Pennsylvanian and Permian rocks is under some artesian pressure, but only one well was observed flowing at the surface. Well 4-16-17daa flowed at a rate of about one-half gallon per minute from the Pillsbury Shale and/or the Zeandale Limestone.
Water Table and Movement of Ground Water
The factors that control the shape and slope of the water table are the topography of the land surface, the underlying bedrock, the transmissibility of the material through which it moves, the relative location of areas of recharge and discharge from the ground-water reservoir, and the relative rate of recharge and discharge.
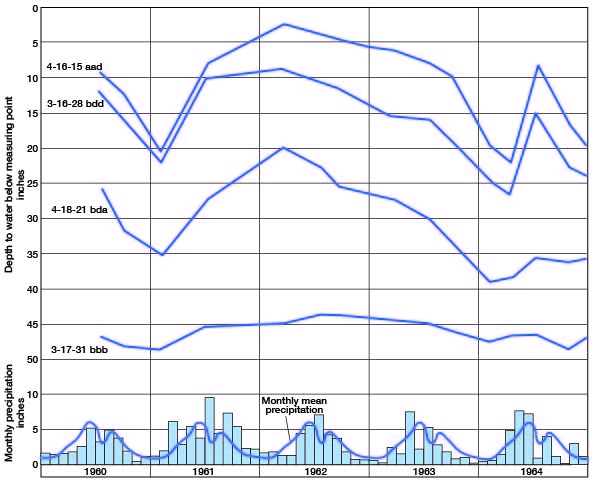
In most areas the water table has the same general shape as the land surface but is more subdued in form and fluctuates in response to gain or loss of water in the aquifer. In some aquifers the fluctuation may be slow and of very little magnitude, but in others the response may be rapid, and fluctuations may be relatively large. Figure 5 shows the hydrographs of four wells in Brown County. Well 3-17-31bbb obtains water from Pennsylvanian limestone, and the other wells obtain water from glacial drift. The hydrographs of wells in the glacial drift are very similar and can be directly related to the weather cycle with lowering water level during periods of drought and rising water level in response to precipitation. Well 3-17-31bbb also can be correlated to the weather cycle, but the response to precipitation and periods of drought is much less than in the wells in glacial drift.
Figure 5--Hydrographs of four wells in Brown County, Kansas, and monthly precipitation and monthly mean precipitation, 1960-64, at Horton, Kansas.
Contours on the water table are shown for part of Brown County on Plate 1. In the western part of the county, water is obtained principally from rocks of Pennsylvanian and Permian ages, and, because the water table is discontinuous or absent in this area, water-table contours are not shown. In the eastern part of the county, the contours are dashed through bedrock areas where the water table is discontinuous. In Brown County the shape of the water table is controlled largely by the topography and to some extent by the underlying bedrock. Most of the major streams have cut through the glacial drift. Discontinuous outcrops of bedrock occur along the valley walls, and alluvial deposits occur in the valley bottoms. Ground water moves in a 90-degree direction from any point on a contour, and it is apparent from the contours on Plate 1 that ground water moves toward and into nearly every stream of any size in the county. Near the valley walls the contours are more closely spaced. This in part indicates steeper topographic conditions but also indicates lower transmissibility of the less permeable bedrock and colluvium through which the water moves. In the upland areas largely underlain by glacial drift (Pl. 1), the contours are more widely spaced, reflecting the flatter upland topography and the more permeable material through which the water moves. In the valley alluvial deposits, water-table conditions exist, and locally this water table is continuous with the water table in the uplands. In those areas where bedrock is present in the valley walls, the water table is discontinuous.
Depth to Water
In Brown County the depth to water in the unconsolidated deposits is generally greatest in areas of the highest topography. The depth to water may determine in a given area the type of well or the type of pump used to obtain the water. In Brown County in areas where the depth to water is less than 25 feet, suction pumps are commonly used. In areas where the depth is below the level for efficient use of suction pumps, force or jet pumps are commonly used. In areas where the water table is shallow, many wells are dug or bored, but in areas where there is an appreciable depth to water, most wells are drilled.
The quantity of water discharged by evaporation and transpiration is closely related to the depth of water. In areas where the water table lies at a depth below the root systems of the vegetation, little water is transpired, and where the depth is more than a few feet, little water is evaporated directly from the zone of saturation or the capillary zone. In Brown County along the edges of many of the valleys (Pl. 2) and on the slope of many of the rounded hills in the drift area, the water table is at or near the surface. Seeps are present locally in these areas and much water is lost by evaporation and transpiration.
The depth to water in wells in Brown County is given in Table 6, and the depth to water in test holes in the glacial drift and valley alluvial deposits is given in Table 7. Depth to water in the glacial drift area is shown in Figure 6. The depth to water in wells given in Table 6 that obtain water from bedrock aquifers does not necessarily indicate the depth at which water was first reached in the well, since water in some of the bedrock aquifers occurs under artesian conditions and rises above the aquifer. Water in glacial drift and in alluvial deposits in the valleys occurs under water-table conditions. The depth to water in the county ranges from 0 to about 80 feet.
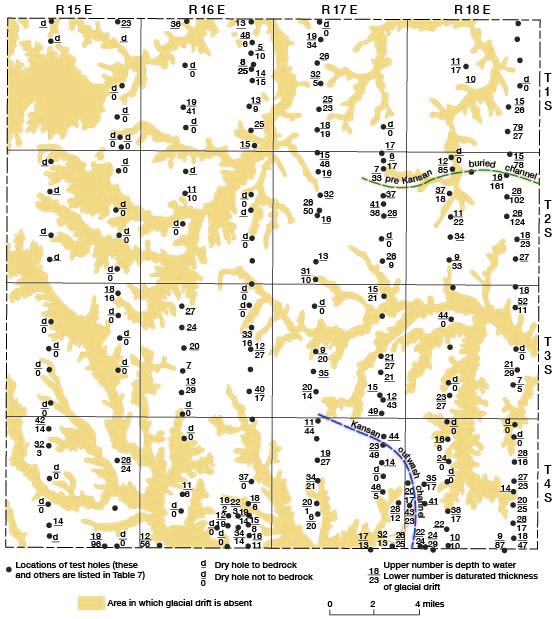
Figure 6--Depth to water and saturated thickness in areas underlain by glacial drift, Brown County, Kansas.
Saturated Thickness of Glacial Drift
Data on the saturated thickness of the glacial drift were obtained from test-hole information and are shown on Figure 6 and given in Table 7. The thickness of saturated material in a water-table aquifer has an important effect on the quantity of water available for use. In wells having materials of equal permeability, the well with the greatest saturated thickness will have the largest potential yield. In Brown County the thickness of saturated material in the glacial drift area is an important factor in yield and reliability of wells from this aquifer. This material, although composed principally of fine materials, contains pockets or lentils of sand and gravel, and where 20 or 30 feet of saturated material is present, one or more of these permeable zones usually can be found in which a dependable water supply can b-. developed. in northwestern and western Brown County and adjacent to many of the streams in eastern Brown County, the drift is thin and dry or contains only a thin zone of saturation. In the eastern part of the county much of the area has a saturated thickness in excess of 20 feet; the maximum (161 feet) is in the northeastern part of the county.
Availability of Ground Water
Ground water is available in Brown County from glacial drift, alluvial deposits in valley areas, and bedrock aquifers. The glacial drift, occurring over much of the upland area of the county, is the most extensive and the most important of the aquifers. It ranges widely in thickness and lithology over short distances and, as these factors affect the availability of water from the drift, yields and dependability of wells in the drift range widely. Where the streams have cut through the drift and erosion has thinned the glacial deposits adjacent to these valleys, the drift is partly drained and is locally dry, resulting in either meager supplies or no water in these areas.
Moderate to large supplies of water are available from the drift in local areas in the county. The largest yields and most extensive supplies occur north of Hiawatha. The drift is thicker in this area and contains more outwash material than elsewhere. Yields may range up to 450 gpm in this area but more commonly are about 100 gpm or less. In an area just west of Everest, moderate supplies of water are available from channel deposits. These deposits are glacial outwash deposits and consist of moderately well-sorted sand and gravel occurring in a north-south trending channel (Fig. 6). Everest obtains water from these deposits in Atchison County. Well 5-18-6dbb was pumped at a rate of 250 gpm with 9 feet of drawdown during tests, but it is usually pumped at about 90 gpm. The channel in which these deposits occur is narrow and the areal extent of the aquifer is limited.
A pre-Kansan buried channel (Fig. 6) trending east-west just south of the line between Townships 1 and 2 in Ranges 17 and 18 is a potential source of moderate to large supplies of ground water. This channel locally contains pre-Kansan sand and gravel deposits in the basal part that are overlain by pro-glacial Kansan lacustrine deposits that are, in turn, overlain by Kansan glacial drift. The lacustrine deposits consist of very fine, bedded sand and some silt, as much as 50 feet thick. This sand is too fine to be successfully screened, and a well cannot be finished in it, but in the areas where it overlies sand and gravel deposits, a well screened in the lower sand and gravel could utilize the water stored in the lacustrine deposits.
In most of Brown County it is difficult to predict yields from wells in the glacial drift because the drift changes so markedly in lithology in short distances, but generally in the eastern part of the county adequate and dependable water supplies for domestic and stock use can be obtained.
Small quantities of water are generally available from alluvial deposits in the stream valleys in Brown County. The materials comprising these deposits are generally poorly sorted and contain much silt and clay intermixed with sand and gravel. This results in wells with small yields. The city of Reserve obtains water from alluvial deposits in Walnut Creek valley, and the city of Robinson obtains water from similar deposits in Wolf River valley. The yield from the municipal wells for these cities is about 20 gpm per well.
The quantity of water available to wells in Brown County from bedrock aquifers ranges widely, depending on local conditions and the individual aquifer. In eastern Brown County many wells obtain water from sandstone beds in the Wabaunsee Group. Yields of wells in sandstone aquifers in the county are generally small but dependable. Numerous springs occur along the walls of the major valleys in eastern Brown County. These springs issue from limestones of the Wabaunsee Group, and where these limestones are overlain by glacial drift, much of the flow of the springs is from water moving downward from the drift. Water moves through, and is discharged from, the limestone joints and solution channels. In a north-south-trending area in the central part of the county, rocks of the Admire Group and the upper part of the Wabaunsee Group are generally poor aquifers. During periods of drought, water shortages occur in wells utilizing these aquifers; locally, however, dependable wells obtain water from thin sandstone beds.
In northwestern Brown County water is obtained from rocks in the lower part of the Council Grove Group. The Grenola Limestone, the Roca Shale, and the Long Creek Limestone Member of the Foraker Limestone are the principal aquifers. Yields ranging up to 250 gpm have been obtained from wells in this area; however, yields less than 100 gpm are common. South from the line between Townships 2 and 3, these aquifers are much less permeable and yields of only a few gpm are obtained. Water from the lower part of the Council Grove Group is generally high in sulfate, with an increase in concentration with depth. Numerous springs yielding water from these aquifers occur in the area west and northwest of Morrill, which obtains water from these aquifers in and just south of the city.
Utilization of Ground Water
In Brown County, ground water is used principally for domestic, stock, and public supplies. Only one industry uses ground water from privately owned wells, and irrigation is not practiced in the county.
Domestic and Stock Supplies
Nearly all water for domestic use and much of the water for stock use in rural areas in the county is obtained from privately owned wells. These range in depth from about 20 to 200 feet. There is no correlation between depth and location or source, for shallow wells and deep wells are found in all parts of the county. Most deep wells are drilled wells, and the shallow wells are about equally divided between dug and drilled wells, although nearly all new wells are drilled. Most domestic and stock wells are pumped at a low rate, although many are capable of yielding more water with the proper pump installation. Yields of domestic and stock wells in the county range from a few gallons per hour to about 40 gpm. In local areas where water shortages periodically occur, cisterns are used for supplemental water supplies. Many ponds have been constructed for stock use.
Public Supplies
Six cities in Brown County have public water supplies; five use ground water and one uses surface water.
The original water system for Everest was built in 1921. In 1934 water was obtained from four wells 60 to 65 feet deep located in the city. Water was obtained from glacial drift and the Bern Limestone. About 1955 better quality water was obtained from outwash channel deposits about 2 miles south of the city. Well 5-18-6dbb is 41 feet deep and was tested at a rate of 250 gpm. The well is normally pumped at a rate of 90 gpm. Analyses of water from well 4-18-29bda in the old well field and from the new well (5-18-6dbb) are given in Table 3. Storage is provided by an elevated steel tank with a capacity of 25,000 gallons. The average daily use is about 25,000 gallons. The water is chlorinated but receives no other treatment.
Hiawatha is the largest city in Brown County. The original water system for the city was built in 1887 when water was obtained from one dug well in a valley about I mile south of town. This well was the only source of supply until about 1912 when a spring (3-17-5aac) was improved and connected to the system. In 1921 another spring (2-16-36ddb) was improved and added to the system. Four additional wells located south of the city were added in the period between 1921 and 1945, and in 1947 two wells (1-17-32ccd and 2-17-5abd) were drilled about 4 miles north of the city. The present supply is obtained from five wells. In 1925 the average monthly pumpage was about 6,000,000 gallons, in 1935 about 7,000,000 gallons, in 1945 about 8,000,000 gallons, and in 1962 about 12,000,000 gallons. Storage is provided by a 500,000-gallon elevated steel tank located in the city and a 60,000-gallon underground concrete reservoir located about 2 miles north of the city. The water is good-quality, calcium-bicarbonate water (Table 3) and is chlorinated but receives no other treatment.
The present Horton water supply is obtained from Mission Lake at the northeast edge of the city. The original water supply was owned by a private water company which obtained water from wells in alluvial deposits in Delaware River valley at the southwest edge of the city. In 1928 the city took over the operation of the water system and drilled wells in the alluvium of Mission Creek at the east edge of the city. These wells and a small lake above the well field were used until Mission Lake was built on this creek in 1935. The average daily use in Horton is about 300,000 gallons. Storage is provided by an elevated steel tank of 250,000-gallon capacity.
The Morrill water supply is obtained from two wells drilled in 1951 about 1 mile south of the city. These wells are about 90 feet deep and yield water from the Grenola Limestone and the Roca Shale. The water is calcium-bicarbonate water and is hard but otherwise of good quality (well 1-15-35dcd, Table 3). The wells are pumped at a rate of about 100 gpm. Formerly the city obtained water from two wells in the southwest part of the city, but this water was high in sulfates (Table 3, well 1-15-26dcd), and the wells are no longer used except in an emergency. This water was obtained from the Long Creek Limestone Member of the Foraker Limestone. The average daily use is about 15,000 gallons and storage is provided by an elevated steel tank of 50,000-gallon capacity. The water is chlorinated but receives no other treatment.
The Reserve water supply is obtained from one well in alluvial deposits in Walnut Creek valley at the east edge of the city. This well (1-17-7cbc) yields about 20 gpm. Another well drilled in 1956 near the original well was used for a short time but is now abandoned. The water is hard but is otherwise of good quality (Table 3). The average daily use is about 10,000 gallons, and storage is provided by an elevated steel tank of 30,000-gallon capacity.
The city of Robinson obtains its water supply from two wells (3-18-4cdc1 and 3-18-9baa) in alluvial deposits in Wolf River valley at the south edge of the city. These wells normally yield about 20 gpm, but they are affected by drought conditions, and yields decline during long periods of drought. Well 3-18-4cac was formerly used for the water supply, but the yield was inadequate, and this well is now used only as a reserve-supply well. Storage is provided by an elevated steel tank having a capacity of 25,000 gallons. The average daily use is about 20,000 gallons.
Industrial Supplies
Only one industry in Brown County uses privately owned wells. The American Telephone and Telegraph Company uses water from wells 3-15-27cab1 and 3-15-27cab2 for cooling purposes in a cable relay station. The wells each produce about 10 gpm from the Foraker Limestone at a depth of about 100 feet. The analysis of a sample of water from well 3-15-27cab1 is given in Table 3.
Temperature
The temperature of ground water ordinarily receives little attention in the discussion of the quality of water or its suitability for use, but many industries prefer ground water for use in cooling systems because of its uniform temperature. The temperature of ground water at relatively shallow depths in an area is generally very near the mean annual temperature for that area (in Brown County, the mean annual temperature is 53.3° F).
Chemical Character of Ground Water
When water comes in contact with rocks that form the crust of the earth, it dissolves a part of the rock material. The type and composition of the rock through which it passes thus determines, to a large degree, the chemical character of such ground water. The more soluble minerals are taken into solution more easily and in greater concentration than the less soluble minerals.
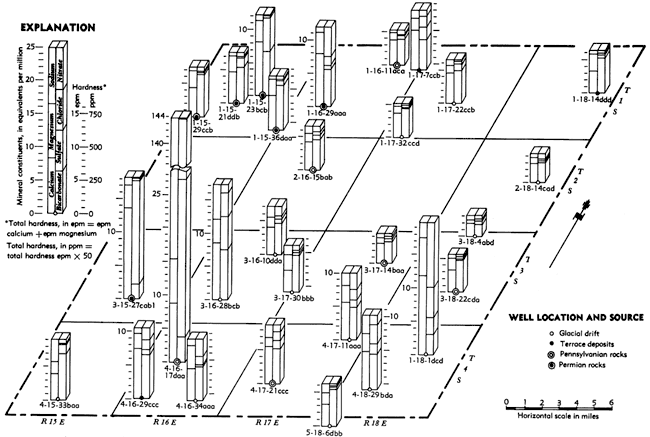
The chemical character of ground water in Brown County is indicated by analyses of 37 samples of water from wells, test holes, and springs given in Table 3 and by 29 analyses shown on Figure 7. Of the 37 analyses, nine are of water from public-supply sources. Although not all the minerals present in the water were determined, those that commonly are present In sufficient quantity to adversely affect the quality of the water for domestic, industrial, and irrigation use are reported.
The mineral constituents listed in Table 3 are reported in parts per million (ppm) by weight. The concentration of minerals in water in equivalents per million (epm) can be computed by multiplying the ppm by the conversion factors given in Table 4. When expressed in equivalents per million, the sum of the anions is equal to the sum of the cations. In an analysis expressed in equivalents per million, unit concentrations of all ions are chemically equivalent.
Table 3--Analyses of water from wells, springs, and test holes in Brown County, Kansas (in parts per million, except as otherwise indicated*). Samples analyzed by H. A. Stoltenberg. One part per million is equivalent to one pound of substance per million pounds of water or 8.34 pounds per million gallons of water. Total hardness of water (carbonate hardness plus noncarbonate hardness): 0-60 ppm, soft; 61-120 ppm, moderately hard; 121-180 ppm, hard; 181+ ppm, very hard.
| Well number |
Date of collection |
Depth of well (feet) |
Geologic source | Temp. (°F) |
Silica (SiO2) |
Iron (Fe) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium (Na) |
Potassium (K) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Nitrate (NO3) |
Dissolved solids (residue at 180° C) |
Hardness as CaCO3 | Specific conductance (micromhos at 25° C) |
pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonate | Non- carbonate |
|||||||||||||||||||
| 1-15-13bcc | 12-21-1953 | Spring | Long Creek Limestone Member of Foraker Limestone | 0.13 | 101 | 34 | 20 | 420 | 37 | 15 | 0.3 | 37 | 454 | 344 | 48 | |||||
| 1-15-21ddb | 12-21-1953 | Spring | Grenola Limestone | .11 | 95 | 29 | 11 | 333 | 65 | 11 | .3 | 28 | 406 | 273 | 83 | |||||
| 1-15-23bcb | 12-21-1953 | 35.0 | Long Creek Limestone Member of Foraker Limestone | .39 | 164 | 39 | 18 | 410 | 242 | 13 | .2 | 2.4 | 684 | 336 | 232 | |||||
| 1-15-26dcd | 10-17-1956 | 90.0 | Long Creek Limestone Member of Foraker Limestone | 17 | 5.3 | 605 | 54 | 46 | 332 | 1,464 | 24 | .6 | 1.8 | 2,378 | 272 | 1,460 | 7.4 | |||
| 1-15-26dda | 12-21-1953 | 65.0 | Grenola Limestone | .33 | 97 | 32 | 13 | 359 | 16 | 16 | .2 | 84 | 438 | 294 | 78 | |||||
| 1-15-29ccb | 4-18-1962 | 50.0 | Grenola Limestone | 54 | 16 | .13 | 91 | 30 | 12 | 0.8 | 393 | 15 | 6.0 | .2 | 32 | 397 | 322 | 28 | 690 | |
| 1-15-35aaa | 12-21-1953 | 44.0 | Grenola Limestone | .62 | 131 | 39 | 10 | 356 | 22 | 44 | .1 | 164 | 588 | 292 | 196 | |||||
| 1-15-35dad | 7-26-1962 | 92.0 | Grenola Limestone and Roca Shale | .09 | 100 | 31 | 3.7 | 354 | 35 | 11 | .1 | 53 | 421 | 290 | 87 | 750 | 7.5 | |||
| 1-15-36daa | 12-21-1953 | 26.0 | Grenola Limestone and Roca Shale | .22 | 104 | 30 | 12 | 415 | 30 | 7.0 | .1 | 35 | 426 | 340 | 44 | |||||
| 1-16-11aca | 4-19-1962 | 78.0 | Wood Siding Formation | 55 | 15 | .54 | 75 | 13 | 10 | .7 | 229 | 31 | 3.0 | .3 | 38 | 299 | 188 | 52 | 520 | |
| 1-16-29aaa | 3-26-1963 | 70.0 | Admire Group | 55 | 16 | .83 | 162 | 38 | 16 | 1.7 | 346 | 28 | 48 | .1 | 248 | 728 | 284 | 276 | 1,150 | |
| 1-17-7ccb | 6-13-1961 | 38.0 | Terrace deposits | 21 | .14 | 120 | 21 | 28 | 276 | 89 | 60 | .2 | 53 | 528 | 226 | 160 | 890 | 7.0 | ||
| 1-17-22ccb | 4-18-1962 | 32.0 | Glacial drift | 54 | 20 | .04 | 91 | 15 | 20 | 1.2 | 300 | 49 | 11 | .5 | 23 | 378 | 246 | 42 | 630 | |
| 1-17-32ccd | 7-17-1962 | 45.0 | Glacial drift | .08 | 74 | 13 | 8.3 | 268 | 13 | 7.0 | .2 | 15 | 282 | 220 | 18 | 490 | 7.4 | |||
| 1-18-14ddd | 4-18-1962 | 15.0 | Alluvium | 55 | 15 | .03 | 65 | 31 | 11 | 2.1 | 317 | 23 | 8.0 | .3 | 26 | 337 | 260 | 30 | 610 | |
| 2-15-27cdc | 4-19-1962 | 115.0 | Grenola Limestone and Roca Shale | 54 | 13 | .04 | 108 | 47 | 27 | 3.1 | 422 | 82 | 28 | .1 | 58 | 574 | 346 | 116 | 960 | |
| 2-16-15bab | 4-19-1962 | 27.5 | Wood Siding Formation | 55 | 12 | .10 | 85 | 16 | 24 | .7 | 283 | 32 | 18 | .2 | 49 | 376 | 232 | 46 | 640 | |
| 2-17-5abc | 3-28-1961 | 97.0 | Glacial drift | 21 | .01 | 67 | 13 | 14 | 256 | 14 | 11 | .2 | 12 | 278 | 220 | 10 | 500 | |||
| 2-18-14cad | 4-19-1962 | 65.0 | Glacial drift | 55 | 21 | .01 | 58 | 9.6 | 14 | 1.2 | 183 | 10 | 10 | .2 | 53 | 267 | 150 | 34 | 450 | |
| 3-15-27cab1 | 3-26-1963 | 100.0 | Foraker Limestone | 55 | 12 | 1.7 | 342 | 51 | 33 | 3.6 | 173 | 932 | 13 | .6 | 2.2 | 1,475 | 142 | 921 | 1,650 | |
| 3-16-10ddd | 3-26-1963 | 50.0 | Glacial drift | 55 | 25 | .26 | 83 | 19 | 34 | 1.2 | 285 | 26 | 22 | .2 | 84 | 435 | 234 | 51 | 670 | |
| 3-16-28bcd | 4-19-1962 | 40.0 | Glacial drift | 54 | 21 | .49 | 169 | 44 | 121 | 2.0 | 449 | 242 | 139 | .4 | 80 | 1,039 | 368 | 234 | 1,660 | |
| 3-17-14baa | 4-19-1962 | Spring | Stotler Limestone | 54 | 11 | .05 | 62 | 10 | 12 | 6.3 | 198 | 28 | 9.0 | .3 | 26 | 262 | 162 | 34 | 440 | |
| 3-17-30bbb | 3-26-1963 | 42.0 | Glacial drift | 54 | 25 | .07 | 66 | 28 | 33 | 1.4 | 346 | 15 | 12 | .1 | 34 | 385 | 280 | 0 | 590 | |
| 3-18-4abd | 11-17-1952 | Spring | Glacial drift | 18 | .14 | 41 | 7.4 | 14 | 115 | 26 | 11 | .3 | 33 | 209 | 94 | 39 | ||||
| 3-18-9baa | 2-1-1960 | 28.7 | Terrace deposits | .26 | 106 | 22 | 19 | 388 | 49 | 16 | .4 | 4.9 | 428 | 318 | 37 | 740 | 7.3 | |||
| 3-18-22cda | 4-18-1962 | 40.0 | Scranton Shale | 54 | 22 | .20 | 67 | 21 | 13 | 1.2 | 290 | 11 | 7.0 | .2 | 33 | 318 | 238 | 16 | 540 | |
| 4-15-33baa | 4-20-1962 | Spring | Glacial drift | 54 | 22 | .05 | 78 | 22 | 76 | .5 | 417 | 67 | 7.0 | .2 | 31 | 509 | 285 | 0 | 830 | |
| 4-16-17daa | 4-19-1962 | 125.0 | Zeandale Limestone and Pillsbury Shale | 5.0 | .99 | 173 | 83 | 2,940 | 24 | 222 | 1,145 | 4,275 | 1.2 | .4 | 8,756 | 182 | 590 | 14,880 | ||
| 4-16-29ccc | 4-19-1962 | 44.0 | Terrace deposits | 53 | 20 | .10 | 147 | 28 | 20 | 1.2 | 393 | 41 | 49 | .1 | 115 | 615 | 322 | 160 | 1,070 | |
| 4-16-34aaa | 3-26-1963 | 69.0 | Glacial drift | 54 | 23 | .15 | 94 | 42 | 22 | 1.4 | 305 | 18 | 40 | .3 | 150 | 541 | 250 | 157 | 860 | |
| 4-17-11aaa | 3-26-1963 | 75.0 | Glacial drift | 55 | 25 | .7 | 113 | 29 | 44 | 1.6 | 298 | 49 | 57 | .2 | 155 | 621 | 244 | 157 | 940 | |
| 4-17-21ccc | 4-20-1962 | 22.0 | Pillsbury Shale | 55 | 19 | .17 | 112 | 16 | 44 | 1.2 | 317 | 58 | 49 | .2 | 53 | 508 | 260 | 86 | 860 | |
| 4-17-28cdd | 7-1-1957 | Lake | Surface water | .95 | 30 | 8.0 | 16 | 134 | 23 | 4.0 | .2 | 3.5 | 156 | 108 | 0 | 273 | 8.0 | |||
| 4-18-10dcd | 4-18-1962 | 35.0 | Glacial drift | 54 | 21 | .08 | 238 | 68 | 54 | 1.6 | 190 | 120 | 160 | .4 | 567 | 1,324 | 156 | 718 | 2,080 | |
| 4-18-29bda | 10-10-1949 | 64.0 | Glacial drift and Bern Limestone | 28 | .08 | 115 | 31 | 68 | 326 | 72 | 102 | .2 | 93 | 708 | 267 | 147 | 8.1 | |||
| 5-18-6dbb | 2-21-1961 | 41.0 | Glacial drift | .02 | 65 | 20 | 31 | 325 | 16 | 7.0 | .3 | 22 | 346 | 244 | 0 | 620 | 7.8 | |||
Table 4--Factors for converting parts per million to equivalents per million.
| Mineral constituent |
Chemical symbol |
Factor |
|---|---|---|
| Calcium | Ca++ | 0.0499 |
| Magnesium | Mg++ | 0.0822 |
| Sodium | Na+ | 0.0435 |
| Potassium | K+ | 0.0256 |
| Carbonate | CO3-- | 0.0333 |
| Bicarbonate | HCO3- | 0.0164 |
| Sulfate | SO4-- | 0.0208 |
| Chloride | Cl- | 0.0282 |
| Nitrate | NO3-- | 0.0161 |
| Fluoride | F-- | 0.0526 |
Figure 7--Chemical character of water in Brown County, Kansas. A larger version of this figure is available.
Chemical Constituents in Relation to Use
The dissolved solids, hardness, iron, fluoride, nitrate, sulfate, and chloride in the samples of water from Brown County are summarized in Table 5 and briefly discussed in the following paragraphs.
Dissolved Solids
The residue after water has been evaporated consists of mineral matter, some organic matter, and water of crystallization. The kind and quantity of minerals in the water determine its usability. Water containing less than 500 ppm of dissolved solids generally is satisfactory for domestic use. Water containing more than 1,000 ppm of dissolved solids may contain enough of certain constituents to cause a noticeable taste or to render it unsuitable for use in some other way (Table 5).
Table 5--Dissolved mineral constituents in ground water, Brown County, Kansas.
| Constituents | Number of samples |
Range in concentration (ppm) |
|---|---|---|
| Dissolved solids | 21 | 500 or less |
| 11 | 501 to 1,000 | |
| 5 | more than 1,000 | |
| Range: 156-8,756 | ||
| Hardness | 0 | 60 or less |
| 2 | 61 to 120 | |
| 3 | 121 to 180 | |
| 32 | more than 180 | |
| Range: 108-1,984 | ||
| Iron | 13 | 0.1 or less |
| 13 | .11 to 0.3 | |
| 9 | .31 to 1.0 | |
| 2 | more than 1.0 | |
| Range: 0.01-5.3 | ||
| Fluoride | 36 | less than 1.0 |
| 1 | 1.0 to 1.5 | |
| 0 | more than 1.5 | |
| Range: 0.1-1.2 | ||
| Nitrate | 21 | 45 or less |
| 9 | 46 to 90 | |
| 3 | 90 to 150 | |
| 4 | more than 150 | |
| Range: 0.4-567 | ||
| Sulfate | 25 | 50 or less |
| 9 | 51 to 250 | |
| 3 | more than 250 | |
| Range: 10-1,464 | ||
| Chloride | 33 | 100 or less |
| 3 | 101 to 250 | |
| 0 | 251 to 1,000 | |
| 1 | 1,001 to 5,000 | |
| Range: 3-4,275 |
Hardness
The hardness of water, the property that generally receives the most attention, is commonly recognized by its effect when soap is used in the water; in a hard water the soap does not lather readily and leaves a curd on the water. Carbonate or "temporary"' hardness is caused almost entirely by calcium and magnesium bicarbonate and may be removed by boiling. Boiling converts the bicarbonate ion to carbonate which is precipitated as calcium carbonate. These constituents combined with bicarbonates and sulfates are the active agents in the formation of scale in steam boilers or other containers in which water is evaporated. The non-carbonate or "permanent" hardness is caused by calcium and magnesium sulfates, nitrates, and some chloride salts and cannot be removed by boiling. Sodium chloride (common salt) does not contribute to hardness in water but is corrosive. Carbonate and non-carbonate hardness react to soap in the same manner. The carbonate hardness and the non-carbonate hardness are given in Table 3.
Water having a hardness less than 60 ppm is considered soft. Water having a hardness between 60 and 120 ppm may be termed moderately hard but for most purposes need not be softened. Water containing more than 120 ppm hardness is generally noticeably hard and for many uses may need to be softened. When municipal supplies are softened, generally, the hardness is decreased to about 100 ppm. In most softening processes, only the carbonate or "temporary" hardness is removed.
Iron
Next to hardness, iron is the most objectionable constituent in natural water. The quantity of iron present may differ greatly from place to place even in the same aquifer. If water contains more than 0.3 ppm of iron in solution, the iron, upon oxidation by exposure to air, may settle out as a reddish sediment. Iron may be present in sufficient quantity to give a disagreeable taste to water, stain cooking utensils and plumbing fixtures, and be objectionable in the preparation of food and beverages. Aeration, followed by settling or filtration, will remove iron from some water, but treatment with chemicals is required for others.
Fluoride
Although the quantity of fluoride present in natural water is relatively small in comparison to other common constituents, the amount present in water used by children should be known. Fluoride in water has been known to cause mottled enamel in the teeth, which may appear in children who, during the formation of the permanent teeth, habitually drink water containing more than 1.5 ppm of fluoride (Dean, 1936). A smaller quantity of fluoride in the drinking water (about 1.0 ppm) is likely to be beneficial by preventing or decreasing the incidence of caries in the permanent teeth of children (Dean, et al., 1941). Fluoride is now added to many public water supplies in quantities sufficient to increase the total fluoride concentration to about 1 ppm.
Nitrate
Large amounts of nitrate in water may cause cyanosis in infants when the water is used for drinking or in the preparation of the food formula. Water containing less than 45 ppm of nitrate is generally regarded as safe, but water containing more than 90 ppm is regarded by the Kansas State Department of Health to be likely to cause severe, possibly fatal, cyanosis if used continuously (Metzler and Stoltenberg, 1950). Nitrate poisoning appears to be confined to infants in their first few months of life. Adults who drink the same water are not affected; however, breast-fed babies of mothers who drink such water may be affected. Also, cows that drink water containing excessive nitrate may produce milk high enough in nitrate to cause cyanosis in infants (U.S. Public Health Service, 1962).
The source of nitrates in natural water is not known. In Kansas, nitrate-bearing rocks sufficiently high in nitrate to contribute the quantities of nitrate which occur in water are not known to exist. Artificial fertilizers and certain legumes may contribute some nitrate in local areas, and seepage from sewage sources or barnyards may also contribute nitrates, but the quantities which have been pumped from wells known to be high in nitrate over several years' period would indicate a renewable source of the nitrates. Nitrate-fixing bacteria may be a principal source of nitrate. Nitrate produced in this manner could be carried down to the aquifer by seepage. Known occurrences of nitrate in Kansas seem to be more common in shallow wells and in aquifers of low transmissibility. The nitrates are commonly more concentrated at or near the water table. At present there is no economical way to remove nitrate from water, but proper construction of a well may materially reduce the amount of nitrate produced by the well. Such a well should be cased and scaled to a point well below the water table and screened and pumped at a low rate from the lowermost part of the aquifer.
Sulfate
Sulfates, when combined with calcium and magnesium, contribute most of the "permanent" hardness to a natural water, and the removal of these salts is both difficult and expensive. Sulfate, especially when combined with magnesium or sodium in excessive amounts (more than 500 ppm) in a domestic water supply, is undesirable because of the laxative effect on persons and animals when the water is used for drinking. A concentration of less than 250 ppm is recommended for human consumption, although a tolerance to the sulfates can be built up.
In Brown County, high concentrations of sulfates are commonly found in water from the Foraker Limestone and less commonly from the Roca Shale and Grenola Limestone (Fig. 7, Table 3).
Chloride
Chloride salts are found in nature in abundance and are dissolved in widely varying.quantities from many rock materials. They are found in sea water and in many ground waters at appreciable depths. Most oil-field brines contain considerable quantities of chloride. Small quantities of chloride have little effect on the suitability of water for ordinary uses. Only when it occurs in sufficient quantity to make the water unpalatable or corrosive to metal pipes or containers is it objectionable. Quantities of chloride permissible in irrigation water vary considerably with the crop being irrigated; however, water containing a high concentration of chloride is generally unsuitable for irrigation.
In the past, removal of chlorides from water has been both difficult and expensive; however in recent years the removal of "salt" from water has been reduced in cost to a point where it is now economically feasible to do this in certain areas where no other water is available-.
In Brown County, chlorides present no problems at the depths ordinarily reached by water-supply wells. Chloride concentrations are generally low in the glacial drift and alluvial aquifers; however, bedrock aquifers, which are low in chlorides at relatively shallow depths, may be high in chlorides at appreciable depth. Well 4-16-17daa yields water very high in chlorides from the Zeandale Limestone or the Pillsbury Shale, but other wells at shallower depths yield water of good quality from the same rocks (Table 3, 5).
Summary of Ground-water Conditions
Ground water is the principal source of supply for public, industrial, and domestic use in Brown County. Five public supplies are obtained from wells and one public supply is from a surface reservoir. Industrial use of ground water is small; only one company obtains water from privately owned wells. Almost all domestic supplies are obtained from wells, but locally, where ground-water shortages occur during periods of drought, cisterns are used for supplemental supplies. Much ground water is used for livestock, but many ponds have been constructed to furnish water for this purpose, also.
Ground water is available from three genera sources in the county. The most extensive and most important aquifer is the glacial drift, which is present in a large part of the area. Relatively small quantities are available from the alluvial deposits in the valleys of the larger streams. Much water is obtained from bedrock aquifers in the area. The largest and most important bedrock supplies are obtained from the Permian rocks in western and northwestern Brown County. In this area little water is available from the glacial drift or the valley alluvium, both of which are thin.
In the glacial drift, water is obtained from sand and gravel lenses that occur throughout glacial till and from sand and gravel that occurs in outwash channels. Moderate to large quantities of water are available in a local area in northeastern Brown County from sand and gravel deposits in the lower part of pre-Kansan lacustrine deposits. Yields up to 450 gpm are obtained from wells in the glacial drift; however, more commonly the yield is less than 100 gpm and locally may be only a few gpm (Table 6).
The alluvial aquifers in the stream valleys are composed of silt, clay, sand, and gravel. These deposits are generally poorly sorted with silt and clay predominating, resulting in low yields from wells.
Small quantities of water are available from sandstone beds in the lower and middle part of the Wabaunsee Group. Springs occur locally along the outcrop of some of the limestones of the lower Wabaunsee. These are "contact" springs and are believed to obtain most of their water from overlying glacial drift. In the area underlain by rocks of the upper Wabaunsee and rocks of the Admire Group, little water is available, and periodic shortages occur in some areas. Rocks in the Council Grove Group of Permian age yield moderate to large quantities of water to wells in western and northwestern Brown County. The Foraker Limestone, Roca Shale, and Grenola Limestone are the principal aquifers in this area. Yields ranging up to 250 gpm are obtained from wells utilizing these combined aquifers; however, yields of less than 100 gpm are more common. South of the line between Townships 2 and 3, these rocks are less permeable, and the yields may be less than 10 gpm.
Water from wells in the county is generally hard but is suitable for most uses. In northwestern Brown County, the water from the Foraker Limestone is generally high in sulfates and locally, water from the Grenola Limestone and the Roca Shale is high in sulfate concentration. Chlorides ordinarily present no problem at the depth reached by most wells in the county, but at appreciable depths the Permian and Pennsylvanian rocks contain water high in chlorides. Water from many wells in the area is high in nitrate. The source of the nitrate in the water is not known. Nitrate concentrations are high in all areas in the county in all types of wells and in all aquifers.
Prev Page--Structure || Next Page--Well Records
Kansas Geological Survey, Geology
Placed on web May 29, 2009; originally published May 1967.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Brown/07_gw.html