Kansas Geological Survey, Open-file Report 2008-32
Part of the Phreatophyte Research Project Project
by
Jesse B. Nippert, Scott E. Spal and Joy K. Ward
KGS Open File Report 2008-32
December 2008
Read the PDF version (172 kB)
This work was conducted 17 km south of Ashland, KS, USA (37° 11' 19"N, 99° 45' 55"W) in riparian habitat adjacent to the Cimarron River. Tamarix first appeared at this location in 1939 following a flood, and is currently the most dominant plant species in the riparian zone. The site overlies a shallow unconfined aquifer that is hydraulically connected to the river. Flow in the Cimarron River in 2006 was intermittent, ending in early June and resuming in mid-September. The most abundant herbaceous species on site included alkali sacaton (Sporobolus airoides (Torr.) Torr.), switchgrass (Panicum virgatum L.), and little bluestem (Schizachyrium scoparium (Michx.) Nash). The site lies within an area mapped as Lincoln-Krier complex, consisting primarily of Lincoln (sandy, mixed, thermic Typic Ustifluvents) and Krier (sandy, mixed, thermic Aeric Halaquepts) soils.
The experimental design consisted of three plots, with two replicate sites per plot designated numerically (1.1, 1.2, 2.1, 2.2, 3.1, 3.2). Each plot was approximately 7.5 ha in size. Tamarix individuals in plot 1 remained uncut. In August 2005, all Tamarix individuals were cut at the ground level in plots 2 and 3, and significant aboveground regrowth (1-1.5 m in height compared to 2-3 m height for uncut Tamarix) had occurred by August 2006. Further details on the experimental layout of this site have been previously described (Butler et al. 2005).
A weather station (Hobo Weather Station logger and sensors, Onset Computer) was installed on site in October 2004. Measurements of air temperature, precipitation, relative humidity, global irradiance, wind speed and direction, and atmospheric pressure were logged at 15-min intervals (Butler et al. 2005).
Six wells were installed in August 2004 among the Tamarix within the aforementioned experimental design. Each well (6.45 m depth, top of screen 1 m below land surface) was equipped with an integrated pressure transducer/datalogger unit (In-Situ MiniTroll, 206.8 kPa) that takes and records pressure-head readings (absolute pressure) every 15 min. Pressure-head readings were corrected for atmospheric pressure, which is measured on site at the same frequency. Water-table position was calculated using the corrected pressure-head measurement and the known elevation of the sensor in the well. Manual measurements of water levels were obtained biweekly during the growing season. Soil samples were taken adjacent to each well to depths of up to 1.2 m and analyzed for particle-size distribution and chemical constituents. Direct-push electrical-conductivity logs to depths of 7 m were obtained at each well site prior to well installation (Butler et al. 2005).
A neutron-probe access tube was installed adjacent to each well in August 2004 and volumetric water content was measured biweekly during the growing season using a Model 503 DR Hydroprobe Moisture Depth Gauge (Campbell Pacific Nuclear). Readings were taken every 15 cm to a depth of 3 m, and standard counts were recorded in the field prior to and after measurements. Volumetric water content (m3m-3) was calculated with an equation based on laboratory calibrations and an adjustment for PVC pipe (Butler et al. 2005).
Repeated physiological measurements of Tamarix were conducted on individuals growing in the uncut plot (1) and one of the cut plots (3). Plant measurements were not performed in plot 2 due to different project objectives previously outlined for the 2006 summer (Butler et al. 2005). Surrounding each well, five Tamarix individuals were randomly selected for repeated measurements throughout the summer. Each individual was within a 5-m radius of the well. We measured the light-saturated photosynthetic rate (Asat), mesophyll CO2 concentration (ci), stomatal conductance to water (gs), and leaf transpiration (E). These gas exchange measurements were performed on new, mature leaves growing in full sunlight using a Li-6400 gas exchange system with a red/blue light source and a CO2 injector (LI-COR, Inc.). Light intensity inside the sample cuvette was 2000 µmol m-2s-1, CO2 concentration was 380 ppm, and the relative humidity was maintained at ambient. All measurements were performed between 11:00-13:00 CST on clear days and corrected for projected leaf area within the gas exchange cuvette using a Li-3100 leaf area meter (LI-COR, Inc.). The same leaf tissue used for gas exchange measurements was dried and analyzed for %N using a Carlo Erba NC-2500 elemental analyzer. Water potential measurements were performed using a Scholander pressure bomb (PMS Instruments) at predawn (4:00-6:00 CST) and mid-day (13:00-15:00 CST) for 2-5 samples per individual per well location. Immediately prior to predawn water potential measurements, the maximum quantum yield of photosystem II (Fv/Fm) was measured on dark-adapted leaves using a modulated chlorophyll fluorometer (Opti- Sciences). For water potential and fluorescence measurements, samples were averaged for each individual prior to analysis. Physiology data were analyzed using analysis of variance with a mixed-effects model, split-plot-repeated measures design in SAS 8.02. In the analysis, date of sampling, treatment (cut versus uncut Tamarix), and their interaction were fixed effects, while well site (1 or 2) within plot (1 or 3) was a random effect. Because measurements were performed on the same individuals over the course of the summer, date of sampling was included as a repeated measure.
On each sampling date, 10-cm subsections of plant stems were collected from each individual, stored in Exetainer vials (Labco) on ice, and then transported to the laboratory within 24 hours for storage at -20 °C. One soil pit was dug adjacent to each well on each sampling date for collection of soil samples at 10- and 30-cm depths. The samples were frozen and stored at -20 °C until analyses could be conducted. Groundwater was collected from each well and stored in Exetainer vials with no headspace at 4 °C. Water was removed from plant and soil samples using cryogenic vacuum extraction (Ehleringer and Osmond 1989). After extraction, the samples were oven-dried at 70 °C for 72 hours to ensure complete vaporization. Extracted water samples were directly equilibrated with a headspace of CO2/He for 24 hours at 30 °C (Epstein and Mayeda 1953). The headspace gas was then analyzed for δ18O on a gas bench connected to a ThermoFinnigan Conflow III interface and Finnigan Delta-plus Continuous Flow Stable Isotope Ratio Mass Spectrometer (IRMS). Within-run variation of sample replicates was 0.19‰, and the laboratory precision associated with the working standard (DITAP) was 0.20‰.
The 2006 growing season was much warmer and drier than the long-term mean conditions for Ashland. Daily maximum and minimum air temperatures, as well as cumulative daily precipitation, have been recorded at Ashland since 1900 (data provided by Mary Knapp, KS state climatologist). The high maximum daily air temperature (Tmax) and low precipitation during 2006 were comparable to the great droughts of the 1930's, the period of the driest and hottest consecutive growing seasons for the last century at this location. In the long-term data set (1900-2006), six years had total growing season precipitation ≤ 251 mm, and 20 years had a mean Tmax ≥ 31.2 °C. However, only two years, 1934 and 1954, had both a mean Tmax ≥ 31.2 °C and precipitation ≤ 251 mm, the conditions recorded over the growing season in 2006.
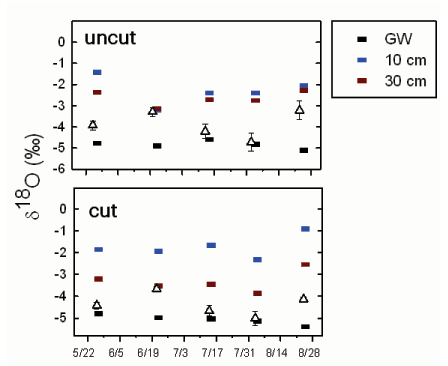
Patterns in the stable isotopic signature of oxygen (δ18O) of water sources used by Tamarix varied by plot treatments during the summer of 2006 (Fig. 1), but differences were minimal. Isotopic signatures in surface soils were heavier in plot 3 compared to plot 1, reflecting the extremely dry soils (i.e., evaporative enrichment) at this location (Butler et al., 2008). Groundwater δ18O varied little between sampling dates and well sites. Given the water sources measured, the xylem δ18O was most similar to the δ18O of groundwater, except during the 6/20 sampling period, when xylem δ18O was most similar to 30 cm soil water. During the 8/24 sampling period, xylem δ18O was between the isotopic composition of the 30 cm soil water and the groundwater.
Fig. 1: Changes in the oxygen stable isotopic signature (δ18O) over the summer from water collected from plant xylem (triangle) and multiple sources. The top panel shows the mean response for the uncut well locations (plot 1; n=10 plants per period). The bottom panel shows the mean response for the previously cut plot (plot 3; n=10 plants per period). Contributing water sources measured included groundwater (GW) and surface water samples collected at 10 cm and 30 cm deep, respectively.

Each of the physiological plant responses we measured did not vary significantly between wells within a given plot (1.1 v. 1.2; 3.1 v. 3.2; p > 0.05). Therefore, statistical analyses were focused on the interaction between plot differences (uncut versus cut) and sampling date during the growing season (Table 1). Gas exchange rates were generally higher in Tamarix individuals in the cut plot (3) compared to the uncut plot (1). Asat rates displayed a similar pattern at all sites, peaking on 6/20 and then remaining relatively stable during the final three sampling dates (Table 2). Patterns of gs were similar to Asat across the entire site, except during the final sampling period when the intercellular CO2 concentrations were also lowest (Table 2). Leaf transpiration (E) had similar trends across all four well sites, with the highest rates observed in the cut Tamarix plot (Table 2). Although E rates were variable among Tamarix individuals measured at a given well site, the mean rate was relatively stable until it declined for the final sampling period. Leaf %N was generally higher in Tamarix individuals in the plot (3) compared to the plot (1), similar to gas exchange data (Table 1, 3). Fv/Fm remained near the theoretical maximum across the growing season, with no significant statistical difference between plots (Table 1, 3).
Table 1: ANOVA results for the physiological responses of Tamarix. Asat is the light-saturated photosynthetic rate, gs is stomatal conductance to water, Fv/Fm is the maximum quantum yield, and predawn and midday water potential (WP) represent minimum and maximum leaf hydrostatic pressure potentials, respectively. The treatment 'plot' compares effects between plots 1 (uncut) and 3 (cut), and 'date' is the sampling period during the summer. An asterisk indicates a significant statistical difference (p < 0.05).
| Effect | ANOVA | |||
|---|---|---|---|---|
| DF | F | p value | ||
| Asat | ||||
| Plot | 1,18 | 10.65 | 0.0043 | * |
| Date | 4,72 | 8.65 | <0.0001 | * |
| Plot*Date | 4,72 | 1.14 | 0.3443 | |
| gs | ||||
| Plot | 1,18 | 6.38 | 0.0212 | * |
| Date | 4,72 | 16.49 | <0.0001 | * |
| Plot*Date | 4,72 | 2.89 | 0.0282 | * |
| Fv/Fm | ||||
| Plot | 1,18 | 0.00 | 0.9772 | |
| Date | 4,72 | 6.65 | 0.0001 | * |
| Plot*Date | 4,72 | 2.08 | 0.0927 | |
| Predawn WP | ||||
| Plot | 1,18 | 22.05 | 0.0002 | * |
| Date | 4,72 | 87.61 | <0.0001 | * |
| Plot*Date | 4,72 | 27.84 | <0.0001 | * |
| Midday WP | ||||
| Plot | 1,18 | 18.74 | 0.0004 | * |
| Date | 4,60 | 41.48 | <0.0001 | * |
| Plot*Date | 3,60 | 3.73 | 0.0158 | * |
| %N (leaf) | ||||
| Plot | 1,18 | 10.21 | 0.005 | * |
| Date | 4,72 | 8.71 | <0.0001 | * |
| Plot*Date | 4,72 | 2.98 | 0.0248 | * |
Table 2: Gas exchange data over the 2006 growing season for each well site. Values are means (±1 SE). Data presented are light-saturated photosynthesis (Asat), mesophyll CO2 concentration (Ci), stomatal conductance (gs) and leaf transpiration (E).
| Asat-µmol CO2-m-2-s-1 | Ci-ppm | |||||||
|---|---|---|---|---|---|---|---|---|
| Date | Well 1.1 | Well 1.2 | Well 3.1 | Well 3.2 | Well 1.1 | Well 1.2 | Well 3.1 | Well 3.2 |
| 5/25/06 | 12.7 (4.0) | 15.3 (1.7) | 26.8 (4.7) | 23.2 (4.9) | 251 (13) | 222 (13) | 275 (13) | 268 (9) |
| 6/20/06 | 26.2 (4.6) | 23.0 (2.8) | 34.8 (2.9) | 24.5 (2.9) | 272 (8) | 256 (9) | 276 (5) | 266 (4) |
| 7/13/06 | 15.7 (1.8) | 16.9 (1.9) | 18.0 (4.3) | 20.2 (2.7) | 255 (7) | 219 (8) | 244 (9) | 235 (4) |
| 8/02/06 | 18.2 (3.5) | 17.3 (3.2) | 26.7 (5.1) | 23.0 (2.2) | 249 (10) | 224 (7) | 245 (11) | 230 (7) |
| 8/24/06 | 12.9 (1.7) | 12.9 (1.7) | 18.1 (2.6) | 21.7 (2.2) | 275 (17) | 275 (17) | 103 (13) | 103 (13) |
| gs-mol H2O-m-2-s-1 | E-mmol H2O - m-2 - s-1 | |||||||
| Date | Well 1.1 | Well 1.2 | Well 3.1 | Well 3.2 | Well 1.1 | Well 1.2 | Well 3.1 | Well 3.2 |
| 5/25/06 | 0.23 (.08) | 0.22 (.04) | 0.73 (.21) | 0.46 (.09) | 9.82 (2.7) | 8.90 (1.3) | 16.46 (3.2) | 11.99 (1.9) |
| 6/20/06 | 0.60 (.17) | 0.41 (.08) | 0.78 (.09) | 0.46 (.06) | 11.66 (2.1) | 10.53 (2.0) | 18.72(1.9) | 12.03(1.5) |
| 7/13/06 | 0.29 (.05) | 0.21 (.02) | 0.31 (.09) | 0.31 (.05) | 13.98 (1.9) | 9.48 (1.3) | 14.93 (3.7) | 15.87 (2.2) |
| 8/02/06 | 0.29 (.05) | 0.23 (.06) | 0.44 (.12) | 0.31 (.04) | 10.42 (1.5) | 8.44 (2.3) | 14.25 (3.0) | 10.86 (1.3) |
| 8/24/06 | 0.13 (.04) | 0.09 (.01) | 0.12 (.03) | 0.12 (.01) | 7.51 (2.0) | 4.76 (0.7) | 6.39 (1.3) | 5.99 (0.5) |
Table 3: Fv/Fm and leaf %N over the 2006 growing season for each well site. Values are means (±1 SE).
| Fv/Fm | %N-leaf (mass) | |||||||
|---|---|---|---|---|---|---|---|---|
| Date | Well 1.1 | Well 1.2 | Well 3.1 | Well 3.2 | Well 1.1 | Well 1.2 | Well 3.1 | Well 3.2 |
| 5/25/06 | 0.802 (0.012) | 0.822 (0.010) | 0.804 (0.010) | 0.798 (0.013) | 1.61 (0.09) | 1.81 (0.14) | 3.07 (0.33) | 1.93 (0.19) |
| 6/20/06 | 0.815 (0.005) | 0.825 (0.004) | 0.830 (0.003) | 0.828 (0.005) | 1.69 (0.15) | 1.51 (0.11) | 2.11 (0.08) | 1.54 (0.13) |
| 7/13/06 | 0.819 (0.007) | 0.829 (0.004) | 0.834 (0.003) | 0.829 (0.006) | 1.37 (0.14) | 1.40 (0.09) | 1.98 (0.15) | 1.60 (0.09) |
| 8/02/06 | 0.821 (0.003) | 0.833 (0.003) | 0.833 (0.003) | 0.833 (0.003) | 1.35 (0.12) | 1.31 (0.22) | 1.47 (0.16) | 1.46 (0.16) |
| 8/24/06 | 0.813 (0.003) | 0.833 (0.003) | 0.833 (0.003) | 0.833 (0.003) | 1.30 (0.13) | 1.41 (0.16) | 1.59 (0.11) | 1.80 (0.14) |
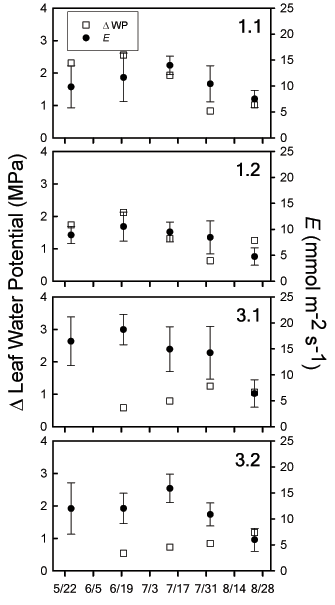
For both cut and uncut Tamarix, leaf water potentials declined from early to midsummer, reaching the lowest potentials at all four sites by mid-July. This period corresponds with the highest Tmax of the summer. For the final two sampling periods (8/02, 8/24), predawn and midday water potentials increased to values similar to those recorded during the early season despite continued hot and dry conditions. The absolute difference between predawn and midday water potentials varied over time and by treatment plot (Fig. 2). The largest absolute differences occurred in the uncut Tamarix, peaking at 6/20 and declining thereafter. Cut Tamarix had the opposite trend, with increasing absolute differences between predawn and midday water potentials after 6/20.
Fig. 2: Changes in leaf water potential calculated as the absolute difference between predawn and midday water potentials for each sampling period (ΔWP: open square: left y-axis). Leaf transpiration rates are presented as means (± 1 SE) for each time period measured (E: filled circles: right y-axis). All results are grouped by well site. Midday water potential measurements were not performed at well sites 3.1 and 3.2 on 5/25 because of a depleted gas supply.
At the beginning of the growing season, the water table was closest to the land surface and the δ18O of xylem water in Tamarix was most similar to groundwater (Fig. 1). This isotopic signature is consistent with the diurnal fluctuations observed at three of the four well sites and the relatively small changes in the volumetric water content profiles (Butler et al., 2008). The shift to a heavier δ18O in xylem water on 6/20 is likely a result of rainfall on 6/16 mixing with and pushing downward isotopically heavier water. The rise in the water table that followed that precipitation event at three of the four well sites demonstrates that water moved downward to the water table in response to rainfall (there was no water in the river at this time). Groundwater δ18O varied little in response to the precipitation because the water sample was taken from a well. Samples from wells are averages representative of some undefined portion of the screened interval and not necessarily representative of conditions at the water table. For example, the water table on 6/20 at well 1.1 was approximately 0.3 m below land surface (top of well screen is 1.0 m below land surface), so the 0.3-m sample should reflect conditions at the water table. The difference between the δ18O of that sample and the sample from well 1.1 demonstrates that water samples from wells may not be the same as point measurements from the water table. At well 3.2, the Tamarix xylem δ18O was always most similar to groundwater, consistent with the lack of water-table response to most precipitation events. During late August, xylem δ18O was heavier than previous samples at all well sites. While these data could be interpreted as an indication of consumption of near- surface sources, the supporting hydrologic data suggest that Tamarix is utilizing a mix of groundwater (as indicated by the diurnal fluctuations at all four wells) and relatively deep vadose zone water (as indicated by the decreases in volumetric water content) [Butler et al., 2008]; the δ18O of the latter reflecting the downward movement of isotopically heavier water in response to rainfall in early August. The similarity between plant and groundwater signatures at wells 3.1 and 3.2 during mid-summer is potentially misleading, as diurnal water-table fluctuations are absent or nearly so at these wells. However, as the water table declined, water left behind in the vadose zone would not be isotopically distinct from groundwater, unless enriched water infiltrates from upper intervals, because fractionation does not occur via root uptake and this region is beyond the zone of evaporative enrichment. Thus, the shift to xylem water with a heavier isotopic signature may be an indication of downward movement of recharging water, and not a switch in water sources. Clearly, more attention needs to be given to the details of subsurface water flow and well construction for reliable interpretations of plant water sources from stable isotopic measurements. In particular, more attention needs to be focused on the vertical distribution of δ18O in the vadose zone and the changes in that distribution with time.
Leaf physiology was relatively constant for Tamarix over the summer, despite low precipitation and progressive increases in Tmax (for similar drought responses also see: Cleverly et al. 1997; Xu et al. 2007). High air temperatures and drought did not cause photoinhibition, as Fv/Fm values remained high (Table 3). Leaf gas exchange rates were also high across the summer (Table 2), as photosynthetic rates remained near the upper range reported for woody C3 species (~20 µmol m-2 s-1; Larcher 1995). Slight seasonal declines in photosynthetic rates corresponded to changes in leaf %N (Table 3), potentially from decreases in mass-based leaf N, shorter leaf life-span and/or shifts in morphological development (Reich et al. 1995). Generally, photosynthetic rates increase linearly with increased leaf N (Field and Mooney 1986; Pons et al. 1989) and Tamarix growing at well locations 3.1 and 3.2 had higher leaf %N than individuals growing at well locations 1.1 and 1.2 (Table 3). Differences in allocation of N to leaves between plots are not surprising, because plot 3 was cut near the end of the 2005 growing season, thereby allowing regrowth in an open canopy with increased light availability during 2006.
Leaf water potential decreased from early to mid-summer, concurrent with the declining water table and drying soil (Fig. 2). Changes in midday leaf water potential are not always related to changes in stomatal closure, because both high rates of leaf transpiration and low soil moisture values can lower leaf water potentials (Reich and Hinckley 1989). It is possible to distinguish between changes in stomatal regulation and hydraulic conductance using predawn water potentials (Reich and Hinckley 1989). If water potential measurements decline proportionally over time, then either stomatal regulation or hydraulic conductance (or both) are determining water potential. However, if midday water potentials decline less than predawn values over time, this result indicates increased stomatal regulation as a driver of water potential values irrespective of changes in hydraulic conductance. In the uncut Tamarix (1.1, 1.2), the absolute difference between predawn and midday water potentials steadily declined from 6/20-8/24 (Fig. 2). This decline confirmed that increased stomatal regulation reduced whole- plant transpiration during the summer drought. This response is consistent with reductions in leaf-level transpiration (Table 2, Fig. 2). The cut Tamarix responded differently, with an increasing absolute difference between predawn and midday water potentials over the same period. Thus, while leaf-level transpiration was decreasing during this period, whole-plant transpiration was likely increasing. This result is possible if Tamarix individuals were rapidly increasing canopy leaf area. Increased canopy leaf area was observed in plot 3, as Tamarix grew from ground-level to 1-1.5-m high by late August. Therefore, while leaf-level responses to changing environmental conditions were similar between cut and uncut Tamarix populations, whole-plant transpiration increased for cut Tamarix during the summer drought.
We thank Luis González, Adam Heitman, and Mary Knapp for analytical assistance. Jim Butler and Don Whittemore significantly contributed to the discussion of the temporal variations in isotopic measurements. This research was supported in part by the Kansas Geological Survey at the University of Kansas, the Kansas Water Resources Institute, the Kansas Water Office, and the Kansas Alliance of Wetlands and Streams. Postdoctoral support for JBN was provided by the National Science Foundation (IOS-0517668), the University of Kansas EEB General Research Fund, and the Kansas Geological Survey.
Butler, J.J., Jr., Whittemore, D.O., and Kluitenberg, G.J. (2005) Ground water assessment in association with salt cedar control--Report on year one activities: Kansas Geological Survey, Open-File Report 2005-19, 29 p. [available online]
Butler, J.J., Jr., Kluitenberg, G.J., and Whittemore, D.O. (2008) Ground water assessment in association with salt cedar control--Report on phase two activities: Kansas Geological Survey, Open-File Report 2008-13, 44 p. [available online]
Cleverly, J.R., Smith, S.D., Sala, A., and Devitt, D.A. (1997) Invasive capacity of Tamarix ramosissima in a Mojave Desert floodplain: The role of drought: Oecologia vol. 111, no. 1, p. 12-18
Ehleringer, J.R., and Osmond, C.B. (1989) Stable Isotopes; in, Pearcy, R.W., Ehleringer, J.R., Mooney, H.A., Rundel, P.W., eds., Plant Physiological Ecology Field Methods and Instrumentation: Chapman and Hall Ltd., London, p. 281-300
Epstein, S., and Mayeda, T. (1953) Variation of 18O content of waters from natural sources: Geochimica et Cosmochimica Acta, vol. 4, no. 5, p. 213-224
Field, C.B., and Mooney, H.A. (1986) The photosynthesis-nitrogen relationship in wild plants; in, Givnish T.J., ed., On the economy of plant form and function: Cambridge Univ. Press, Cambridge, p. 22-55
Larcher, W. (1995) Physiological Plant Ecology, 3rd ed.: Springer, Berlin, p. 85-86
Pons T.L., Schieving, F., Hirose, T., and Werger, M.J.A. (1989) Optimization of leaf nitrogen allocation for canopy photosynthesis in Lysimachia vulgaris; in, Lambers, H, Cambridge, M.L., Honings, H., Pons, T.L., eds., Causes and consequences of variation in growth rate and productivity of higher plants: SPB Academic, The Hague, p. 175-186
Reich, P.B., and Hinckley, T.M. (1989) Influence of pre-dawn water potential and soil-to-leaf hydraulic conductance on maximum daily leaf diffusive conductance in two oak species: Functional Ecology, vol. 3, no. 6, p. 719-726
Reich, P.B., Kloeppel, B.D., Ellsworth, D.S., and Walters M.B. (1995) Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species: Oecologia, v. 104. no. 1, p. 24-30
Xu, H., Li, Y., Xu, G.Q., and Zou, T. (2007) Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation: Plant Cell and Environment, vol. 30, no. 4, p. 399-409
Kansas Geological Survey, Geohydrology
Placed online Jan. 5, 2009
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Hydro/Publications/2008/OFR08_32/index.html