Kansas Geological Survey, Public Information Circular (PIC) 21
Prev. Page--Start of the Report || Next Page--Salt Production
![]()
![]()
![]()
![]()
Kansas Geological Survey, Public Information Circular (PIC) 21
Prev. Page--Start of the Report ||
Next Page--Salt Production
Before Kansas was settled by Europeans, salt marshes and salt springs were used by wildlife, Native Americans, and early travelers. Wild animals of the plains-especially bison, deer, antelope, and elk-obtained salt from places known as licks. Salt licks, or salt flats, are areas where saline ground water reaches the surface and then evaporates during dry times, leaving salt on top of the ground. Native Americans, explorers and hunters, and early ranchers obtained salt by evaporating water collected from salt springs. Early hunters visited the salt marshes to jerk buffalo meat. They would either evaporate brine or dip the meat in pools of strong brine and then dry it in the sunshine or by a fire.
Salt was commercially manufactured in Kansas as early as 1863 at the Osawatomie Salt Works in Miami County in eastern Kansas. Brine, produced from five wells that tapped a saline aquifer, was evaporated in 17 kettles (each holding 30 gallons) set in a single furnace; the salt sold locally for $1.40 per bushel (a bushel of salt weighs 56 pounds). About the same time, salt was produced in Republic County at the Tuthill marsh by scraping the salt scale from the marsh, dissolving it in water, and then siphoning off the clear brine. The brine was then evaporated to recover the salt. Salt was sold in nearby Seapo, and hauled to Manhattan, where it sold for as much as ten cents per pound. Other early salt plants were established at Solomon in Dickinson County in 1867.
Rock salt was discovered in central Kansas in 1887 by speculation companies organized to drill for coal, gas, oil, or any valuable minerals they might encounter. Although the main objectives were not found in commercial quantities, drillers repeatedly encountered beds of salt several hundred feet thick occurring at shallow depths (500 to 1,000 feet). Rock salt was first mined in Hutchinson in 1888, using solution mining (a mining method that pumps water down wells to dissolve the salt), when two wells and a salt factory were put into operation. By 1891, underground mines (using a mining method similar to underground coal mining) produced salt at Lyons, Kingman, and Kanopolis. Underground mining began at Hutchinson in 1923 when the Carey Salt Mine was officially dedicated.
Salt is called an evaporite mineral because it is formed by the evaporation of water. Gypsum and anhydrite are also evaporites. Sea water contains salt in solution. When sea water evaporates, salt is deposited on the ocean floor. During most of the Permian Period, shallow seas covered what is now Kansas. Sea level fluctuated-sometimes the land was exposed and a terrestrial environment existed; at other times, mudstones (shale) and limestones were deposited in a normal marine setting.
When the Hutchinson Salt Member formed, however, the climate was hot and dry, and the sea was restricted to central Kansas-probably an isolated arm of the main ocean to the south, or cut off entirely. The rate of evaporation exceeded the inflow of water, and as evaporation continued and the salt content of the water increased, thick layers of salt built up on the sea bottom. It takes about 80 feet of sea water to produce a foot of salt, so it must have taken thousands of years to accumulate the thick salt deposits of central Kansas. Over time, the salt layer was covered by younger rocks.
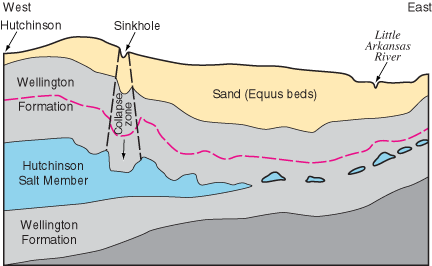
The eastern edge of the Hutchinson Salt Member is actively being eroded, or dissolved, by contact with ground water (fig. 4). This area, where the salt is closest to the surface, is known as the dissolution front. Because salt is so easily dissolved in water, outcrops at the surface are not present in Kansas.
Figure 4--Generalized cross section from Hutchinson to the Little Arkansas River west of Newton (in Harvey County) showing dissolution of the Hutchinson Salt Member and related subsidence features. red line represents deformation of beds within the shale.
Salt also has other unusual characteristics. It is plastic-that is, it will flow and move very slowly, over long periods of time, when it is under pressure, as it is underground. This plasticity allows salt to slowly heal or cover up small fractures or openings in the salt beds.
Prev. Page--Start of the Report || Next Page--Salt Production
Why are the Quivira Salt Marshes Salty?
Kansas Geological Survey, Public Outreach
Web version July 2002
http://www.kgs.ku.edu/Publications/pic21/pic21_2.html