Prev Page--Utilization || Next Page--Geologic Formations
Quality of Water
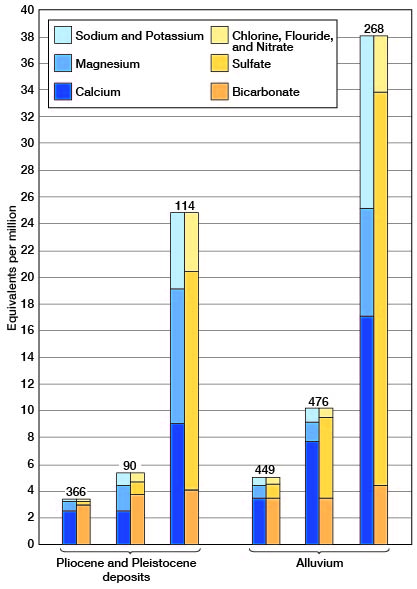
The chemical character of the well waters in Finney and Gray counties is shown by the analyses of water from 55 representative Wells given in tables 20 and 21. Figure 19 shows graphically the chemical character of typical waters from the Ogallala formation and undifferentiated Pleistocene deposits and from the alluvium.
All of the samples of water were collected by me except those from public supply wells. F.O. Holmes, chemist, analyzed the samples in the Water and Sewage Laboratory of the Kansas State Board of Health. The analyses show only the dissolved mineral content of the waters and do not in general indicate the sanitary condition of the waters. The chemical constituents of the waters were determined by the methods used by the U.S. Geological Survey.
Figure 19--Analyses of typical waters from the Ogallala formation and undifferentiated Pleistocene deposits and from the alluvium in Finney and Gray counties, Kansas.
Chemical Constituents in Relation to use
The following discussion of the chemical constituents of ground water has been adopted in part from publications of the United States Geological Survey.
Total dissolved solids--The residue left after a natural water has evaporated consists of rock materials, with which may be included some organic material and some water of crystallization. Waters containing less than 500 parts per million of dissolved solids are generally entirely satisfactory for domestic use, except for the difficulties resulting from their hardness and, in some areas, excessive iron corrosiveness. Waters having more than 1,000 parts per million are generally not satisfactory, for they are likely to contain enough of certain constituents to produce a noticeable taste or to make the water unsuitable in some other respects.
The water from four of the wells (388, 392, 497, and 538) sampled contained less than 200 parts per million of dissolved solids. The waters from 36 of the 55 wells sampled contained between 200 and 500 parts per million of dissolved solids, the waters from three wells (12, 55, and 476) contained between 500 and 1,000 parts, the waters from eight wells (114, 125, 141, 165, 233, 336, 338, and 441) contained between 1,000 and 2,000 parts, and the water from four wells (268, 297, 317, and 349) contained more than 2,000 parts. The greatest concentration of dissolved solids--3,197 parts per million--was found in the water from well 297.
Hardness--The hardness of water, which is the property that generally receives the most attention, is most commonly recognized by its effects when soap is used with the water in washing. Calcium and magnesium cause virtually all the hardness of ordinary waters. These constituents are also the active agents in the formation of the greater part of the scale formed in steam boilers and in other vessels in which water is heated or evaporated.
In addition to the total hardness, the table of analyses shows the carbonate hardness and the noncarbonate hardness. The carbonate hardness is that due to the presence of calcium and magnesium bicarbonates. It is almost completely removed by boiling. In some reports this type of hardness is called temporary hardness. The noncarbonate hardness is due to the presence of sulfates or chlorides of calcium and magnesium, and it cannot be removed by boiling and has sometimes been called permanent hardness. With reference to use with soaps, there is no difference between the carbonate and noncarbonate hardness. In general, the noncarbonate hardness forms harder scale in steam boilers.
Water having a hardness of less than 50 parts per million is generally rated as soft, and its treatment for the removal of hardness under ordinary circumstances is not necessary. Hardness between 50 and 150 parts per million does not seriously interfere with the use of water for most purposes, but it does slightly increase the consumption of soap, and its removal by a softening process is profitable for laundries or other industries using large quantities of soap. Waters in the upper part of this range of hardness will cause considerable scale on steam boilers. Hardness above 150 parts per million can be noticed by anyone, and if the hardness is 200 or 300 parts per million it is common practice to soften water for household use or to install cisterns to collect soft rain water. Where municipal water supplies are softened, an attempt is generally made to reduce the hardness to 60 or 80 parts per million. The additional improvement from further softening of a whole public supply is not deemed worth the increase in cost.
The ground waters of Finney and Gray counties are practically all hard--only two samples (analyses 500 and 526) had less than 150 parts per million of hardness. The other 53 samples ranged in hardness from 152 to 1,641 parts per million. Sixteen of the samples analyzed had between 150 and 200 parts per million of hardness, 24 samples had between 200 and 500 parts, eight samples had between 500 and 1,000 parts (analyses 55, 114, 125, 141, 165, 336, 338, and 441), and five samples had more than 1,000 parts (analyses 233, 268, 297, 317, and 349). The greatest concentration of hardness was 1,641 parts per million (analysis 297).
Iron--Next to hardness, iron is the constituent of natural waters that in general receives the most attention. The quantity of iron in ground waters may differ greatly from place to place, even though the waters are derived from the same formation. If a water contains much more than 0.1 part per million of iron, the excess may separate out and settle as a reddish sediment. Iron, which may be present in sufficient quantity to give a disagreeable taste and to stain cooking utensils, may be removed from most waters by simple aeration and filtration, but a few waters require the addition of lime or some other substance.
Twenty-one of the 55 samples of water from Finney and Gray counties contained only 0.1 part per million or less of iron, and all but four of the samples had less than 3 parts per million. The water from well 500 had 3.9 parts per million of iron, the water from well 449 had 4.0 parts, that from well 472 had 4.2 parts, and that from, well 73 had 5.2 parts.
Fluoride--Although determinable quantities of fluoride are not so common as fairly large quantities of the other constituents of natural waters, it is desirable to know the amount of fluoride present in waters that are likely to be used by children. Fluoride in water has been shown to be associated within the dental defect known as mottled enamel, which may appear on the teeth of children who drink water containing fluoride during the period of formation of the permanent teeth. It has been stated that waters containing 1 part per million or more of fluoride are likely to produce mottled enamel, although the effect of 1 part per million is not usually very serious (Dean, 1936). If the water contains as much as 4 parts per million of fluoride, 90 percent of the children exposed are likely to have mottled enamel, and 35 percent or more of the cases will be classified as moderate or worse.
Of the 55 samples of ground water collected in this area, 28 contained 1.0 part per million or more of fluoride; of these, 20 contained 1.0 to 2 parts, seven contained 2 to 3 parts, and the sample of water from well 500 contained 4.2 parts. The fluoride content of samples of water from the Ogallala formation and from undifferentiated Pleistocene deposits is shown in figure 21 and discussed on pages 170 and 171.
Water for irrigation--The suitability of water for use in irrigation is commonly believed to depend mainly on the total quantity of soluble salts and the ratio of the quantity of sodium to the total quantity of sodium, calcium, and magnesium. The quantity of chloride may be large enough to affect the use of the water, and in some areas other constituents, such as boron, may be present in sufficient quantity to cause difficulty. In a discussion of the interpretation of analyses with reference to irrigation in southern California, Scofield (1933) suggests that if the total concentration of dissolved salts is less than 700 parts per million there is not much probability of harmful effects in irrigation use, but if it exceeds 2,100 parts per million there is a strong probability of damage to either the crops or the land, or both. Water containing less than 50 percent sodium (the percentage being calculated as 100 times the ratio of the total bases, in equivalents) is not likely to be injurious, but if it contains more than 60 percent its use is inadvisable. Similarly, a chloride content of less than 142 parts per million is not objectionable, but more than 355 parts per million is undesirable.
The waters from 13 of the wells sampled in this area contained more than 700 parts per million of dissolved solids, and the waters from wells 268, 297, 317, and 349 contained more than 2,100 parts per million. All but two of the samples contained less than 50 percent sodium (including potassium); these two (analyses 500 and 526) contained more than 60 percent. Analyses 500 and 526, however, are of samples of water from wells that tap the Dakota formation and are not in an area where water is pumped for irrigation use. All of the samples analyzed contained less than 355 parts per million of chloride, and most of them contained less than 142 parts.
According to the limits given by Scofield, it would seem that the waters from wells 268, 297, 317, and 349 are unsuited for irrigation use because of their large concentration of dissolved solids. Under certain conditions waters of this type may be injurious. Scofield (1933) recognizes, however, that no hard and fast limits can be adopted because the harmfulness of irrigation water is so dependent on the nature of the land, the crops, the manner of use, and the drainage.
The use of ground water for irrigation has been practiced in Finney and Gray counties for more than 25 years with no reported harm to the soils or crops.
Sanitary Considerations
The analyses of water given in the tables show only the amounts of dissolved mineral matter in the water and do not indicate the sanitary quality of the water. The water in a well may contain mineral matter that imparts an objectionable taste or odor and yet be free from harmful bacteria and entirely safe for drinking. On the other hand, the water in a well may be clear and pleasant to the taste and yet contain harmful bacteria.
It is well recognized that every precaution should be used to protect domestic and public water supplies from pollution by organic material. Much of the population of Finney and Gray counties is dependent on private water supplies from wells, and it rests chiefly with the drillers and individual well-owners to observe precautions in constructing wells to insure a safe and wholesome water supply. It is obvious that a well should not be located where there are possible sources of pollution nor where surface water can descend to the water table. The drainage from cesspools and privies is particularly dangerous. Every well should be so constructed as to seal off all surface water. As a general rule, dug wells are more subject to contamination from surface water than are drilled wells, owing mainly to the fact that generally they are not effectively sealed at the surface.
Natural Softening of Waters in the Dakota Formation by Base-exchange
The soft sodium bicarbonate waters from the Dakota formation are believed to result from a natural softening process in which calcium bicarbonate water has exchanged part of its calcium and magnesium for sodium by a base-exchange process. The chemical character of two samples (analyses 500 and 526) of water from the Dakota formation and one sample (analysis 499) from the Ogallala formation, which directly overlies the Dakota over much of southern Gray County, is shown by figure 20. Waters in the Ogallala formation are typically moderately hard calcium and magnesium bicarbonate waters (p. 169), and it seems probable that before the natural softening by base-exchange took place the waters of the Dakota formation were somewhat similar in quality to the waters of the Ogallala formation.
Figure 20--Analyses of waters from the Ogallala formation (499) and the Dakota formation (500 and 526) illustrating natural softening of the water in the Dakota formation by base-exchange. Numbers refer to analyses in table 21.
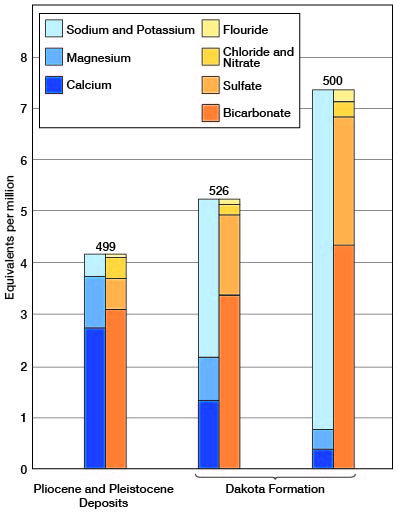
Similar natural softening by base-exchange has been described by Renick (1924) in Rosebud county, Montana, and by Piper (1933, pp. 85-87) in southwestern Pennsylvania. Renick (1924, p. 69) believes that in the Montana area leverrierite is the principal mineral causing the natural softening of the water, but recognizes that other hydrated aluminum silicates, such as kaolin, feldspar, and mica, also are capable of exchanging wholly or in part their sodium and potassium for other bases. The base-exchange silicates which are active in southwestern Pennsylvania are thought by Piper (1933, pp. 85, 86) to be the clay-forming minerals of the montmorillonite and hydro-mica groups.
The principal base-exchange silicates active in the Dakota formation in the area under consideration presumably are the clay-forming minerals of the montmorillonite group, although other minerals such as feldspar, mica, and kaolin might also be taking part in the exchange. No samples of the Dakota from this area have been analyzed, but Norman Plummer, ceramist for the State Geological Survey of Kansas, collected samples of clay from outcrops of the Dakota formation in central and northern Kansas and had them analyzed by R.E. Grim, petrographer, Illinois Geological Survey. Plummer (personal communication) reports that in general the clays analyzed contained about 75 percent kaolinite, and that the remaining 25 percent consisted chiefly of illite (group of mica-like clay minerals) but also in small part of clay minerals of the montmorillonite group. Base-exchange is thought to have occurred at places where the water-bearing sandstones are in contact with clay beds or by contact with the admixed clay in the sandstones themselves.
Prev Page--Utilization || Next Page--Geologic Formations
Kansas Geological Survey, Geology
Web version April 2002. Original publication date Dec. 1944.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/General/Geology/Finney/08_qual.html