Kansas Geological Survey, Open-file Report 2001-61
by
Donald O. Whittemore and P. Allen Macfarlane
Prepared for
Pawnee-Buckner Subbasin Water Resources Management Program,
Division of Water Resources,
Kansas Department of Agriculture
KGS Open-file Report 2001-61
In 1967, a farmer discovered that ground water in the alluvial aquifer of a small stream valley in western Kansas had become saline. The ground water in the area of concern is principally used for irrigation. Subsequent investigations by state agencies and a consulting company failed to conclusively explain the reason for the salinity. Potential sources that were suggested included saltwaters associated with petroleum production and saline ground waters migrating upwards along faults or unplugged core holes associated with petroleum exploration. In 1999, the Kansas Department of Agriculture requested the assistance of the Kansas Geological Survey in determining the salinity source.
The saline water in the alluvial aquifer has contained chloride and sulfate concentrations as high as about 800 mg/L and 1,400 mg/L, respectively, since the salinity discovery. Geochemical methods based on sulfate/chloride and bromide/chloride ratios and mixing curves revealed that the primary source of salinity fits concentration of natural sources of dissolved salts in the ground waters of the alluvial aquifer by the process of evapotranspiration mainly associated with irrigation in the Buckner Creek valley. The natural source of the sulfate is probably dissolution of gypsum in Cretaceous shale underlying part of the alluvial aquifer. The natural source of most of the chloride is probably ground water in the Dakota aquifer also underlying part of the alluvial valley. Assuming 78-80 percent consumption of the alluvial ground water used during irrigation, the original dissolved solids in the water would be concentrated by a factor of 4-5 during an irrigation season. The saline return flow from the irrigation can recharge the alluvial aquifer and thereby increase the ground-water salinity. High nitrate concentrations in the alluvial aquifer water in comparison with low values in the Dakota aquifer also fit the return recharge as the primary cause of the salinity.
Higher ground-water salinities appear to be associated with years of low precipitation and streamflow. During some summers, the streamflow is zero or near zero. Pumping from the alluvial aquifer would be expected to reduce ground-water discharge to the stream and exacerbate low streamflow conditions. Small or zero baseflow to the stream means that saline ground water is not flushed from the alluvial aquifer but accumulates in the valley. Periods of greater precipitation result in less irrigation water use, greater recharge to the alluvial aquifer, and flushing of some of the salinity buildup through discharge to the stream. Thus, the salinity of the alluvial ground waters could decrease following extended wet periods and increase during long dry periods.
In 1967, a farmer discovered that ground water in the alluvial aquifer of Buckner Creek valley in Hodgeman County had become saline. The well that yielded the high-chloride ground water is on the Smith property approximately 5 miles east of Jetmore. The salinity source was initially believed to have been an oil-field brine storage pond. Other irrigation wells were constructed on surrounding properties after 1967. These wells also yielded mineralized ground water even though investigation by the Kansas Department of Health and Environment (KDHE) determined that some of these wells are upgradient of the brine storage pond. The KDHE suggested that possible sources included 1) an old saltwater storage pond, 2) artesian upwelling of saltwater from the Cedar Hills Sandstone from an unplugged core hole, fault zone, or from the Dakota Formation subcrop along the south wall of the Buckner Creek Valley, or 3) an abandoned, plugged well that had disposed saltwater into the Cedar Hills Sandstone and that was located in the same general vicinity as the brine pond (KDHE Request for Proposal, in Total Environmental Services and Technology, 1993).
In 1990, the KDHE selected a consulting company, Total Environmental Services and Technology (TEST), to perform an investigation of the chloride contamination associated with the irrigation wells. The study site was named Smith/Wasko after the property owners of the irrigation wells with the high-chloride ground water. The objectives of the investigation were to assess the ground-water quality and identify possible sources of the chloride contamination. TEST conducted two electromagnetic conductivity surveys and an earth resistivity survey, drilled 18 test holes and installed monitoring wells in 5 of the borings, collected ground-water samples, and obtained analyses of the samples. The study found high sulfate and nitrate concentrations in the ground water as well as elevated chloride content. The summary of the TEST report implied that gypsum in the bedrock could be the source of the high sulfate in the water. TEST also indicated that pumping of the mineralized ground water from the main part of the aquifer for irrigation could explain the higher chloride content in the upper portion of the ground-water column in one test hole than in the lower part of the column. However, TEST concluded that their investigation "did not find any one particular source of brine contamination that could be contributing to the increased chloride concentrations of the south Smith irrigation well" (TEST, 1993).
The irrigators communicated their continued concerns with the high-chloride ground waters in the alluvial aquifer to staff of the Oil and Gas Conservation Division of the Kansas Corporation Commission (KCC) and of the Pawnee-Buckner Subbasin Water Resources Management Program, Division of Water Resources (DWR) in the Kansas Department of Agriculture. The KCC investigated the records for oil and gas wells and saltwater disposal wells in the area to determine whether there was a possibility of oil-brine contamination. In 1999, the DWR staff asked the Kansas Geological Survey (KGS) for assistance in more clearly identifying the salinity source(s). The DWR collected ground-water and surface-water samples from the study area and sent them to the KGS for analysis and geochemical identification of salinity source(s). The DWR cooperated in the study with KCC staff, who collected oil-brine samples for the KGS to analyze. The DWR also measured water levels in selected wells in the study area.
The site area identified by KDHE for the Smith/Wasko investigation by TEST was the NW of Section 1 and the NE of Section 2, T. 23 S., R. 23 W (Figure 1). The cooperative DWR and KGS study included this site and the surrounding area extending along 5 miles of the Buckner Creek valley from about 2 miles east of Jetmore to 3 miles southwest of Hanston. The KGS examined the existing geologic, hydrologic, and water-quality information for the study area and interpreted the new water-level and water-quality data. Based on this data, the KGS was able to determine the most probable sources of the high concentrations of chloride, sulfate, and nitrate in the ground waters and the mechanisms controlling these sources.
Figure 1--Location of the Smith/Wasko study area (bounded by dashed line on west, south, and east sides and by road on north side) within the Buckner Creek valley. The study is the NW Sec. 4 and NE Sec. 2, T. 23 S., R. 23 W.

The surface extent of the alluvial aquifer of Buckner Creek gradually widens from approximately 0.5 mile to 1 mile from the west to the east end of the 5-mile study area. The Smith/Wasko site is a rectangle 0.5 mile north-south by 1 mile east-west that extends along the alluvial valley with its south side on the south valley wall and the north side just south of Buckner Creek. Based on the test hole data of TEST (1993), the thickness of the alluvial aquifer in the Smith/Wasko area ranges up to about 67 ft in the NE of the NE of Section 2. In the main part of the alluvial aquifer where the thickness of the fluvial sediments is generally from nearly 50 ft up to 65 ft, the uppermost 40 ft of deposits are composed of silts and clays. The more permeable portion of the aquifer is poorly sorted sands and gravels that lie along the top of the bedrock and below the lower permeability silts and clays. The thickness of the sands and gravels ranges from about 10 to 30 ft in the main part of the alluvial aquifer.
The bedrock underlying the alluvial valley consists of Cretaceous shales and sandstones of the Dakota Formation and Graneros Shale. The contact between the Graneros and underlying Dakota (Macfarlane, et al., 1996) passes through Section 2, the section in which the west half of the Smith/Wasko area is located. The Graneros Shale is composed primarily of noncalcarcous shale, although calcareous and non-calcareous sandstone and siltstone lie in the middle part at many localities (Zeller, 1968). Selenite, the crystalline form of gypsum (CaSO4 ⋅ 2H2O), is abundant in weathered parts of the shale. The upper part of the Dakota consists of fine to medium grained sandstones encased in a matrix of mudstone (Macfarlane et al., 1998).
The regional direction of ground-water flow in the Dakota aquifer is to the east-northeast (Macfarlane, et al., 1998). The water-level elevation contours tend to wrap around the Buckner Creek valley in Hodgeman County, indicating that ground water discharges from the Dakota aquifer to the alluvial aquifer in the study area. Local recharge can enter the sandstones and fractured mudstone of the Dakota Formation in the uplands of the study area and combine with regional Dakota flow to discharge into the alluvial aquifer in the valley. Small amounts of local recharge could possibly enter fractured and weathered Graneros Shale along the valley walls of Buckner Creek and also discharge to the alluvial valley.
The DWR measured water levels in 16 wells on March 9, 2000 (Table 1). The values for the depth of the water level below land surface were converted to water-level surface elevations using the topographic map for the area. The water-level surface elevations were contoured as shown in Figure 2. The contours show the general eastward flow of ground water within the alluvial aquifer down the Buckner Creek valley. The contours wrap around Buckner Creek, indicating a component of ground-water flow toward the creek; the creek serves as the discharge zone for the ground water.
Table 1--Water-levels measured in wells in Buckner Creek valley by the Division of Water Resources on March 9, 2000.
| File No. | Name | Legal description | Aquifer | Hold, ft | Cut, ft | Depth to water, ft | Measuring point above land surface | Depth below land surface, ft | Measuring point description |
|---|---|---|---|---|---|---|---|---|---|
| 17105 | 22S-23W-35 DCA | Alluvial | 32.0 | 7.86 | 24.14 | 1.00 | 23.14 | ||
| 9684 | 23S-23W-03 CBD | Alluvial | UTM | ||||||
| HG72 | 23S-23W-03 ACC | Alluvial | 27.0 | 2.28 | 24.72 | 3.10 | 21.62 | ||
| 8407 | Salmans | 22S-23W-36 DBC | Alluvial | 30.0 | 3.15 | 26.85 | 1.00 | 25.85 | |
| 38764 & 36647 | Salmans | 22S-23W-36 DDC | Alluvial | 40.0 | 12.82 | 27.18 | 2.10 | 25.08 | |
| HG61 | 22S-23W-35 DDD | Alluvial | 350 | 5.35 | 29.65 | 0.10 | 29.55 | Hole under flap, NE corner pumpbase | |
| 3529 | 23S-23W-02 ABB 2 | Alluvial | 31.0 | 7.54 | 23.46 | 1.90 | 21.56 | ||
| 3529 original well | 23S-23W-02 ABB 1 | Alluvial | 41.0 | 18.48 | 22.52 | 1.05 | 21.47 | ||
| HG71 North | 22S-23W-35 CDD 2 | Alluvial | 31.0 | 5.38 | 25.62 | 1.20 | 24.42 | ||
| HG71 South | 22S-23W-35 CDD 1 | Alluvial | 28.0 | 2.60 | 25.40 | 0.20 | 25.20 | ||
| 28015 | 23S-23W-03 ABD | Alluvial | 29.0 | 3.04 | 25.96 | 1.00 | 24.96 | Hole under NE corner of pumpbase | |
| 15437 | Bristow | 23S-23W-04 AAD | Alluvial & Dakota | 40.0 | 11.79 | 28.21 | 0.50 | 27.71 | |
| 13388 | Hoagland | 23S-23W-04 DCA | Alluvial & Dakota | 35.0 | 7.37 | 27.63 | 0.80 | 26.83 | Remove plug in NE side pumpbase |
| 17105 & HG 62 | 22S-23W-35 CAA | Alluvial | 30.0 | 0.71 | 29.29 | 0.85 | 28.44 | Hole under flap, NE side pumpbase | |
| 17105 & HG 64 | 22S-23W-35 DBC | Alluvial | 30.0 | 2.56 | 27.44 | 0.35 | 27.09 | Hole under flap in pumpbase | |
| HG65 | Salmans | 23S-23W-06 BAA | Alluvial | 35.0 | 5.36 | 29.64 | 1.50 | 28.14 | |
| 28176 | Salmans | 23S-22W-06 AAB | Alluvial | 30.0 | 1.99 | 28.01 | 0.50 | 27.51 | Hole under flap in pumpbase |
| 19130 | Salmans | 22S-22W-31 CDC | Alluvial | 34.0 | 7.34 | 26.66 | 2.25 | 24.41 | Meas Tube, NE side of pumpbase |
| HG50 | Salmans | 22S-22W-31 DCC | Alluvial | 29.0 | 5.06 | 23.94 | 0.35 | 23.59 | Hole under flap in pumpbase |
| 36641 | Salmans | 22S-23W-36 CDB | Alluvial | 30.0 | 3.98 | 26.02 | 2.00 | 24.02 | Top of Casing |
| 17819 | Lupfer | 23S-23W-01 BAC | Alluvial | 30.0 | 7.3 | 22.6 | 1.50 | 21.1 | Meas Tube, SE side of pumpbase |
| KGS-Obs | 23S-22W-07 DAA | Dakota | 105.0 | 16.38 | 88.62 | 1.30 | 87.32 | Top of Casing |
Figure 2--Configuration of the water-table surface in the alluvial aquifer of Buckner Creek from water-level measurements made in March 2000. A larger version of this figure is available as an Acrobat PDF file.
The U.S. Geological Survey currently maintains a stream-gaging station on Buckner Creek near Burdett. The gaging station is located at the bridge approximately 5 miles to the northeast of Hanson or about 10 miles to the northeast of the Smith/Wasko area. Streamflow gaging began in October 1995 at the Buckner Creek station. Two gaging stations exist on Pawnee Creek, one is located west of Burdett at a bridge to the north of the Buckner Creek station and upstream of the confluence with Buckner Creek, and the other is located at Rozel, downstream of the entrance of Buckner Creek. The gaging record for the Pawnee River near Burdett started in 1981 and for the station at Rozel began in 1925. Up to September 1995, the station for the Pawnee River downstream of Buckner Creek was approximately 4 miles to the east of the current station at Rozel. Since the start of gaging of Buckner Creek, the annual recorded streamflow has been greater than that in the Pawnee River near Burdett and has comprised the majority of the flow in the Pawnee River at Rozel. Thus, the record of the Pawnee River at Rozel can serve as a suitable proxy for the general streamflow pattern expected before 1995 in Buckner Creek.
Streamflow in Pawnee River and Buckner Creek is characterized by periods of low or no baseflow interspersed with brief episodes of high flow during substantial precipitation events. The hydrograph of daily streamflow in the Pawnee River at Rozel from 1960 to 2000 (Figure 3) illustrates this characteristic. Most of the time the flow in the river was less than a few cfs (cubic ft/sec) during this period, and many times there was no flow. The long-term annual discharge of the Pawnee River also exhibits a highly variable pattern of very high and low flow years (Figure 4). The linear regression through the discharge data for the Pawnee River appears to indicate a long-term decline in flow. In general, the variability of the runoff is so great that it obscures the relatively smaller changes in baseflow. The same discharge data in Figure 4 are plotted on a log axis in Figure 5 to emphasize the long-term changes in low flow years. From 1925 to the early 1960's, the lowest flow years had annual discharges that were all greater than 7,000 acre-ft. From the mid-1960's to the present, there have been 7 years with a total discharge of less than 3,000 acre-ft. In 1991, there was no flow in the Pawnee River at Rozel. The annual flow of Buckner Creek parallels (at somewhat lower values) that of the Pawnee River based on the available record (Figure 6).
Figure 3--Daily streamflow of Pawnee River at Rozel during 1960-2000.
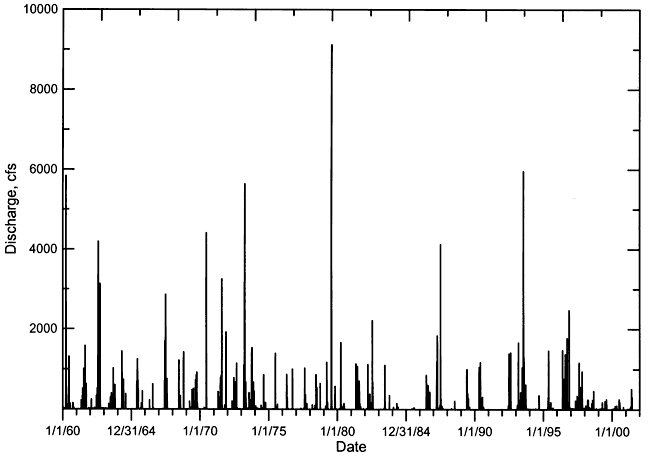
Figure 4--Annual flow of the Pawnee River at Rozel during 1925-2000. The dashed line represents the linear regression for the data.
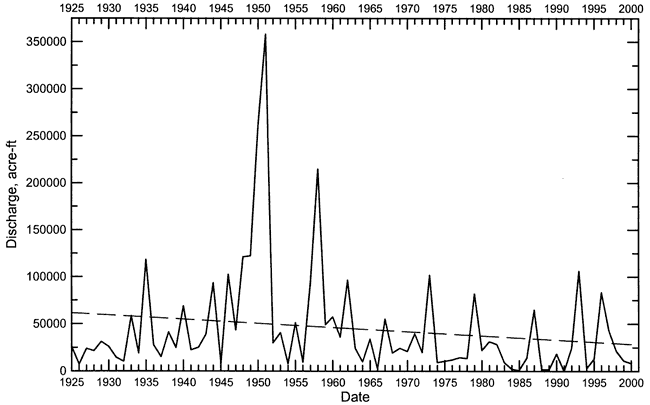
Figure 5--Annual flow of the Pawnee River at Rozel plotted on a log axis for the period 1925-2000. The dashed line represents the linear regression for the data.
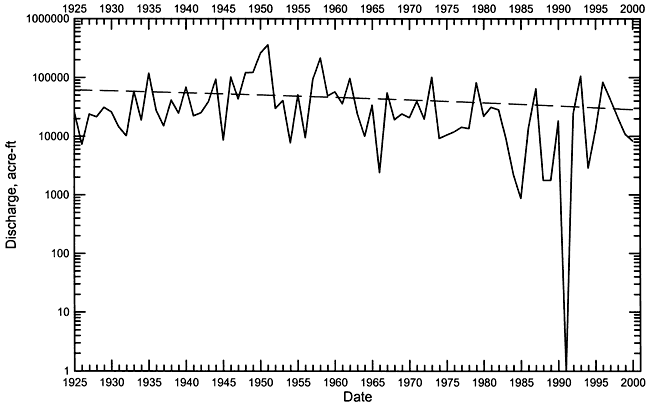
Figure 6--Annual flow of the Pawnee River at Rozel during 1995-2000, and of Buckner Creek near Burdett during 1996-2000.
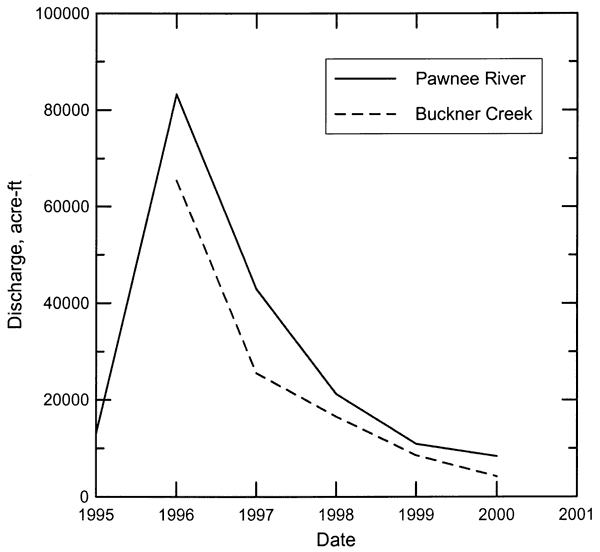
Table 2 lists chemical data for well-water samples that were collected during the KDHE and TEST investigation in 1990 and 1991. The data were printed in the TEST (1993) report. The quality of the data was checked by calculating the charge-balance error for the analyses for which there are complete determinations of major constituent ions (Ca, Mg, Na, K, HCO3 [converted from alkalinity], Cl, SO4, NO3). The charge balance of a water is zero, i.e., the equivalent concentrations of the cations (positively charged species dissolved in water) equal the equivalent concentrations of the anions (negatively charged species). For waters containing substantial concentrations of major constituents, a charge balance error of less than 5% is a very good analysis, 5-10% is acceptable, 10-15% is fair, 15-20% is poor, and >20% is generally unacceptable. A problem resulting from high errors is that it is difficult to know which constituent values are good and which are poor. This makes it difficult to use some of the data for salinity trends and identification, especially when ratios are used. However, if examination of data with large charge balance errors reveals a pattern of error in one or two constituents, it may be possible to use some of the other major constituent concentrations for qualitative purposes.
Table 2--Chemical data for well waters collected from the Smith/Wasko study site by TEST during 1990 and 1991.
| Well identification |
Sample interval |
Sample pumping time |
Laba | Sample date m/d/yr |
Sample time 24 hr |
pH units |
SiO2 mg/L |
Ca mg/L |
Mg mg/L |
Na mg/L |
K mg/L |
Total alkalinity (CaCO3) mg/L |
Cl mg/L |
SO4 mg/L |
NO3-N mg/L |
F mg/L |
TDS measd. mg/L |
TDS sum mg/L |
Charge balance error % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. Wasko Irrigation | 30 min. | KDHE | 6/16/90 | 10:45 | 33.0 | 312 | 58.2 | 266.6 | 17.6 | 311 | 296 | 696 | 12.7 | 0.93 | 1923 | 5.3 | |||
| S. Wasko Irrigation | 2 hrs. | KDHE | 6/16/90 | 12:20 | 33.1 | 316 | 57.8 | 265.2 | 18.4 | 313 | 299 | 747 | 12.2 | 0.96 | 1979 | 3.6 | |||
| S. Wasko Irrigation | KDHE | 6/16/90 | 20:00 | 33.0 | 313 | 58.5 | 268.0 | 18.7 | 312 | 300 | 734 | 12.7 | 0.98 | 1970 | 4.0 | ||||
| S. Wasko Irrigation | KDHE | 6/17/90 | 20:00 | 33.6 | 310 | 58.5 | 268.3 | 17.7 | 312 | 292 | 722 | 12.1 | 0.99 | 1944 | 4.6 | ||||
| S. Wasko Irrigation | 53.5 hrs. | KDHE | 6/18/90 | 17:40 | 33.2 | 305 | 57.6 | 263.7 | 16.2 | 312 | 285 | 795 | 11.6 | 1.00 | 1995 | 1.5 | |||
| N. Wasko Irrigation | 30 min. | KDHE | 6/16/90 | 10:50 | 30.0 | 289 | 41.3 | 163.8 | 19.3 | 343 | 255 | 597 | 0.82 | 0.60 | 1605 | -0.3 | |||
| N. Wasko Irrigation | KDHE | 6/16/90 | 12:30 | 32.0 | 300 | 43.0 | 172.8 | 19.6 | 343 | 254 | 611 | 0.73 | 0.59 | 1642 | 1.3 | ||||
| N. Wasko Irrigation | 9.5 hrs. | KDHE | 6/16/90 | 19:45 | 33.0 | 313 | 44.9 | 179.3 | 20.2 | 339 | 253 | 598 | 0.60 | 0.60 | 1648 | 4.0 | |||
| N. Wasko Irrigation | KDHE | 6/17/90 | 20:10 | 32.3 | 302 | 43.2 | 170.3 | 18.9 | 343 | 246 | 582 | 0.65 | 0.60 | 1604 | 2.8 | ||||
| N. Wasko Irrigation | 54 hrs. | KDHE | 6/18/90 | 17:20 | 32.3 | 297 | 42.5 | 167.0 | 19.4 | 342 | 248 | 579 | 0.66 | 0.60 | 1594 | 2.1 | |||
| N. Smith Irrigation | 30 min. | KDHE | 7/6/90 | 16:00 | 7.4 | 36.7 | 194 | 29.6 | 144.3 | 13.0 | 273 | 129 | 303 | 21.3 | 0.74 | 1109 | 7.0 | ||
| N. Smith Irrigation | 1.5 hrs. | KDHE | 7/6/90 | 17:00 | 7.6 | 35.4 | 190 | 29.4 | 141.0 | 12.9 | 255 | 126 | 307 | 22.2 | 0.68 | 1094 | 6.9 | ||
| N. Smith Irrigation | KDHE | 7/6/90 | 23:30 | 7.2 | 35.5 | 237 | 33.8 | 111.8 | 15.1 | 366 | 130 | 375 | 7.79 | 0.67 | 1193 | 3.5 | |||
| N. Smith Irrigation | 23 hrs. | KDHE | 7/7/90 | 14:45 | 7.1 | 34.0 | 218 | 31.3 | 98.9 | 13.6 | 362 | 128 | 360 | 4.10 | 0.71 | 1120 | 0.7 | ||
| S. Smith Irrigation | 30 min. | KDHE | 7/6/90 | 15:30 | 7.3 | 37.8 | 524 | 93.4 | 454.3 | 28.6 | 310 | 786 | 1200 | 23.3 | 0.88 | 3414 | 0.4 | ||
| S. Smith Irrigation | 2 hrs. | KDHE | 7/6/90 | 17:0 0 | 7.2 | 36.9 | 525 | 93.3 | 451.9 | 27.9 | 315 | 588 | 1250 | 23.7 | 0.88 | 3268 | 4.5 | ||
| S. Smith Irrigation | 8.5 hrs. | KDHE | 7/6/90 | 23:30 | 7.1 | 36.1 | 519 | 93.8 | 464.8 | 29.0 | 321 | 573 | 1360 | 21.9 | 0.91 | 3366 | 3.1 | ||
| S. Smith Irrigation | 23 hrs. | KDHE | 7/7/90 | 14:45 | 7.1 | 34.4 | 497 | 90.0 | 452.4 | 27.1 | 316 | 565 | 1290 | 19.6 | 0.97 | 3233 | 3.1 | ||
| E. Smith Irrigation | 30 min. | KDHE | 7/8/90 | 9:40 | 7.3 | 34.9 | 277 | 43.1 | 264.5 | 18.6 | 315 | 391 | 579 | 0.80 | 1798 | 1.5 | |||
| E. Smith Irrigation | 2 hrs. | KDHE | 7/7/90 | 11:40 | 7.2 | 34.2 | 277 | 42.9 | 265.3 | 19.7 | 317 | 383 | 604 | 0.78 | 1817 | 1.0 | |||
| MW-1 | QAS | 10/4/90 | 13:30 | 270 | 43.5 | 179.0 | 234 | 234 | 560 | 5.19 | 0.68 | 1650 | 1500 | 3.8 | |||||
| MW-2 | QAS | 10/4/90 | 14:05 | 250 | 50.9 | 119.0 | 212 | 222 | 412 | 27.5 | 1.08 | 1460 | 1354 | 2.5 | |||||
| TH-3 | QAS | 10/2/90 | 15:03 | 478 | 56.6 | 212.0 | 328 | 700 | 236 | 11.7 | 0.70 | 2400 | 1982 | 8.8 | |||||
| TH-3 | Top | KDHE | 10/18/90 | 16:50 | 7.4 | 35.4 | 302 | 39.6 | 211.3 | 22.4 | 363 | 842 | 296 | 7.85 | 0.52 | 2002 | -13.0 | ||
| TH-3 | Bottom | KDHE | 10/18/90 | 16:55 | 7.1 | 46.2 | 27801 | 68.4 | 246.6 | 27.0 | 284 | 657 | 908 | 17.7 | 1.02 | 4983 | 56.1 | ||
| TH-4 | QAS | 10/2/90 | 13:30 | 384 | 51.9 | 193.0 | 345 | 309 | 900 | 3.32 | 0.97 | 1880 | 2110 | -3.5 | |||||
| TH-5 | QAS | 10/3/90 | 13:57 | 862 | 169.0 | 318.0 | 552 | 650 | 1100 | 1.49 | 0.73 | 1 4850 | 3487 | 15.6 | |||||
| TH-6 | OAS | 10/2/90 | 14:00 | 374 | 62.0 | 108.0 | 479 | 359 | 573 | 3.36 | 0.83 | 2280 | 1829 | -4.7 | |||||
| TH-7 | QAS | 10/4/90 b | 11:45 b | 1050 | 108.0 | 1215.0 | 421 | 621 | 489 | 19.4 | 0.78 | 2860 | 2872 | 31.3 | |||||
| TH-8 | QAS | 10/13/90 | 10:35 | 450 | 92.7 | 319.0 | 240 | 494 | 1000 | 40.0 | 0.95 | 2480 | 2728 | 2.1 | |||||
| TH-8 | Bottom | KDHE | 10/18/90 | 17:10 | 7.1 | 72.9 | 1047 | 79.9 | 281.4 | 27.2 | 279 | 505 | 1120 | 26.1 | 1.09 | 3417 | 23.7 | ||
| TH-9 | QAS | 10/12/90 | 21:40 | 196 | 38.8 | 197.0 | 234 | 295 | 780 | 0.35 | 1.47 | 1100 | 1700 | -14.7 | |||||
| TH-9 Split | Bottom | QAS | 10/17/90 | 16:00 | 1570 | 92.7 | 240.0 | 179 | 343 | 600 | 3.01 | 1.27 | 3018 | 57.9 | |||||
| TH-9 | Bottom | KDHE | 10/17/90 | 16:00 | 7.2 | 45.5 | 2555 | 45.4 | 183.3 | 13.3 | 304 | 325 | 647 | 3.63 | 1.25 | 4014 | 66.1 | ||
| TH-10 | QAS | 10/13/90 | 14:21 | 420 | 90.4 | 330.0 | 256 | 334 | 812 | 25.5 | 1.01 | 2210 | 2304 | 12.9 | |||||
| TH-10 | KDHE | 10/18/90 | 16:40 | 7.1 | 90.6 | 1263 | 84.1 | 307.5 | 26.6 1 | 266 | 318 | 1360 | 14.6 | 1.33 | 3676 | 32.3 | |||
| TH-11 | QAS | 10/14/90 | 11:10 | 437 | 99.4 | 358.0 | 256 | 584 | 1050 | 42.5 | 0.86 | 2800 | 2921 | -0.7 | |||||
| TH-11 | Top | KDHE | 10/18/90 | 17:20 | 7.2 | 34.4 | 449 | 66.2 | 280.0 | 20.3 | 308 | 614 | 1310 | 30.2 | 0.76 | 3093 | -12.2 | ||
| TH-11 | Bottom | KDHE | 10/18/90 | 17:25 | 7.1 | 73.4 | 2722 | 93.8 | 331.5 | 30.6 | 287 | 590 | 1310 | 30.3 | 0.8 | 5458 | 51.2 | ||
| TH-12 | QAS | 10/14/90 | 11:37 | 260 | 51.4 | 1231.0 | 256 | 353 | 742 | 0.57 | 1.19 | 1480 | 1845 | -5.2 | |||||
| TH-13 | QAS | 10/18/90 | 11:19 | 392 | 76.0 | 290.0 | 234 | 523 | 1100 | 16.0 | 1.11 | 1990 | 2643 | -5.8 | |||||
| TH-13 | Bottom | KDHE | 10/18/90 | 16:00 | 7.1 | 54.8 | 2012 | 83.9 | 305.1 | 25.9 | 293 | 662 | 969 | 9.91 | 1.15 | 4334 | 46.0 | ||
| TH-14 | QAS | 10/18/90 | 16:23 | 618 | 125.0 | 291.0 | 245 | 372 | 753 | 16.5 | 1.15 | 2560 | 2430 | 25.5 | |||||
| TH-14 | Bottom | KDHE | 10/18/90 | 15:50 | 7.1 | 112.2 | 1455 | 131.3 | 314.9 | 32.0 | 282 | 468 | 1550 | 17.4 | 1.15 | 4311 | 31.0 | ||
| TH-15 | QAS | 10/19/90 | 10:06 | 290 | 66.0 | 238.0 | 267 | 419 | 790 | 14.0 | 1.32 | 1600 | 2076 | -6.2 | |||||
| TH-15 | Top | KDHE | 10/19/90 | 15:05 | 7.2 | 32.3 | 292 | 59.7 | 265.2 | 14.1 | 277 | 425 | 725 | 12.3 | 1.20 | 2036 | -2.1 | ||
| TH-15 | Bottom | KDHE | 10/19/90 | 15:00 | 7.4 | 63.9 | 500 | 55.2 | 222.8 | 13.8 | 263 | 415 | 712 | 12.4 | 1.26 | 2196 | 10.4 | ||
| MW-16 | QAS | 10/19/90 | 9:46 | 572 | 115.0 | 303.0 | 290 | 464 | 910 | 15.7 | 0.94 | 4370 | 2658 | 14.0 | |||||
| MW-17 | QAS | 1/23/91 | 11:00 | 654 | 71.3 | 433.0 | 389 | 462 | 1080 | 5.04 | 0.73 | 1980 | 3007 | 14.1 | |||||
| MW-17 | QAS | 11/4/90 | 9:43 | 253 | 35.1 | 274.0 | 276 | 163 | 550 | 8.50 | 0.47 | 1500 | 1529 | 11.4 | |||||
| MW-18 | QAS | 1/23/91 | 14:30 | 494 | 73.9 | 296.0 | 339 | 440 | 836 | 1.34 | 0.76 | 1700 | 2400 | 9.2 | |||||
| MW-18 | QAS | 11/4/90 | 13:42 | 330 | 67.9 | 247.0 | 286 | 201 | 900 | 0.22 | 0.68 | 2080 | 1969 | 4.8 | |||||
| Jetmore Water Supply | QAS | 1/23/91 | 13:50 | 171 | 24.3 | 35.0 | 336 | 86.9 | 116 | 1.86 | 0.59 | 664 | 668 | 3.2 | |||||
| S. Wasko Irrigation | KDHE | 10/18/90 | 16:30 | 7.2 | 32.6 | 346 | 65.1 | 279.0 | 16.9 | 307 | 420 | 924 | 9.85 | 0.15 | 2311 | -2.6 | |||
|
aKDHE = Kansas Department of Health and Environment; QAS = Quality Analytical Services bComposite sample collected at 11:45 and 1:30 on 10/4/90 and at 13:30 on 10/5/90 |
|||||||||||||||||||
There are 32 sample analyses by the KDHE and 22 analyses by the QAS laboratory in Table 2. Three of the KDHE analyses have charge-balance errors in the range 10-15% and 7 analyses have errors >20%. Four of the QAS analyses have charge-balance errors within 10-15%, one analysis has an error within 15-20%, and 3 analyses have errors >20%. All of the analyses with errors >15% are positive, meaning that the equivalent cation concentrations are substantially greater than those of the anions.
Table 3 lists analyses of waters collected from the Smith/Wasko study site during 1993-1998. The analyses do not include all of the major, charged dissolved species. Thus, a charge-balance error cannot be calculated. The analyses were by the KDHE and Oil and Gas Conservation Division of the Kansas Corporation Commission (KCC).
Table 3--Chemical data for well waters collected from the Smith/Wasko study site during 1993-1998.
| Well identification | Sample interval | Laba | Sample date m/d/yr |
Sample time 24 hr |
pH units |
Total alkalinity (CaCO3) mg/L |
Cl mg/L |
SO4 mg/L |
NO3-N mg/L |
F mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| MW-1 | KDHE | 4/30/93 | 12:30 | 7.3 | 413 | 270 | 1140 | 5.74 | 0.53 | |
| MW-2 | KDHE | 4/30/93 | 12:50 | 7.5 | 230 | 177 | 336 | 0.31 | 1.71 | |
| MW-16 | KDHE | 4/30/93 | 14:00 | 7.2 | 291 | 506 | 1080 | 23.9 | 1.02 | |
| MW-17 | Top at 33' | KDHE | 4/30/93 | 14:10 | 7.6 | 358 | 320 | 778 | 13.4 | 0.73 |
| MW-17 | Bottom at 65' | KDHE | 4/30/93 | 14:15 | 7.2 | 323 | 663 | 1120 | 11.4 | 0.84 |
| MW-18 | Bottom at 65' | KDHE | 4/30/93 | 13:45 | 7.1 | 331 | 370 | 895 | 13.3 | 1.02 |
| MW-18 | Top at 33' | KDHE | 4/30/93 | 13:30 | 7.3 | 288 | 264 | 808 | 11.5 | 1.30 |
| MW-2 | SWL - 34.7' | KCC | 11/16/95 | 210 | ||||||
| MW-16 | SWL - 29.9 | KCC | 11/16/95 | 490 | ||||||
| MW-17 | SWL - 29' | KCC | 11/16/95 | 530 | ||||||
| MW-18 | SWL - 30.4' | KCC | 11/16/95 | 420 | ||||||
| MW-1 | SWL - 25.66' | KCC | 4/14/98 | 370 | ||||||
| MW-2 | SWL - 28.17' | KCC | 4/14/98 | 190 | ||||||
| MW-16 | SWL - 24.56' | KCC | 4/14/98 | 470 | ||||||
| MW-17 | SWL - 23.81' | KCC | 4/14/98 | 490 | ||||||
| MW-18 | SWL - 21.83' | KCC | 4/14/98 | 430 |
Examination of the data in Tables 2 and 3 for inconsistencies in concentrations reveals that the calcium values often appear to be much too great. The higher calcium concentrations in these tables (>800 mg/L) also would not be expected to be possible in the waters because they would substantially exceed the solubility of calcium carbonate, even in the presence of the levels of sulfate and chloride in the waters. The set of analyses for the irrigation wells in June and July 1990 are very good; the high charge-balance errors are in the monitoring well analyses. In general, the chloride concentrations appear to be relatively consistent in the data with high charge-balance errors. The KCC determinations of chloride are expected to be good values based on water analyses by the KGS for splits of samples collected and analyzed by the KCC for other projects. The sulfate values in Tables 2 and 3 appear to be consistent enough for qualitative use.
The KGS labs strive for <2% error and nearly all our complete analyses data has errors <3%. The chloride concentrations for the data set may not be too far in error for most of the samples because there are field determinations for many that appear to be consistent with the lab values. Some of the Ca values are clearly far from correct. I will include the analysis of errors in the report data in a report that I will eventually produce for the Subbasin. The errors occur in data from both the QAS and KDHE labs.
The DWR collected ground-water samples from 6 observation wells and one windmill at the Smith/Wasko site on April 9, 2000 (Table 4, Figure 7). All of the monitoring wells are screened in the lower part of the alluvial aquifer of the Buckner Creek valley. The windmill well is probably in shallow alluvial sediments where Rock Creek enters the southern part of the alluvial valley of Buckner Creek. The DWR also sampled Rock Creek at 3 locations; the first site was nearly 2 miles upstream of the Smith/Wasko site, the second about 0.3 mile upstream of the site, and the third at the eastern boundary of the site. The KCC collected two oil brines produced from oil wells, one within the Smith/Wasko site and the other about one mile south of the site. The 10 water samples and 2 brine samples were collected in polyethylene bottles and sent to the KGS for analysis. The water samples and brine samples were kept cool in an ice chest during transport to the KGS.
Table 4--Information for water samples collected as part of the KGS-DWR study of the Smith/Wasko study area in 2000.
| Sample site | Sample type | Legal location | Sample date |
Sample time |
Collecting agency |
Collector name | Field Sp.C. μS/cm |
KGS lab number |
|---|---|---|---|---|---|---|---|---|
| MW-1 | Well water | 23S-23W-2ABBC | 3/9/00 | 12:30 | DWR | Iona Branscum | 3490 | 000030 |
| MW-2 | Well water | 23S-23W-2ACBB | 3/9/00 | 11:15 | DWR | Iona Branscum | 2200 | 000031 |
| MW-2 (2nd sample) | Well water | 23S-23W-2ACBB | 3/9/00 | 13:00 | DWR | Iona Branscum | 000032 | |
| MW-1 6 | Well water | 23S-23W-2ADAD | 3/9/00 | 10:10 | DWR | Iona Branscum | 4060 | 000033 |
| MW-1 7 | Well water | 23S-23W-1BAC | 3/9/00 | 15:15 | DWR | Iona Branscum | 000034 | |
| MW-1 8 | Well water | 23S-23W-2ABD | 3/9/00 | 15:00 | DWR | Doug Doubek | 3500 | 000035 |
| SE Windmill | Well water | 23S-23W-1BDC | 3/9/00 | 13:15 | DWR | Iona Branscum | 2200 | 000036 |
| Rock Creek 1 | Stream water | 23S-23W-11DCC | 3/9/00 | 16:35 | DWR | Iona Branscum | 000037 | |
| Rock Creek 2 | Stream water | 23S-23W-1CDC | 3/9/00 | 15:55 | DWR | Iona Branscum | 4060 | 000038 |
| Rock Creek 3 | Stream water | 23S-23W-1ABC | 3/9/00 | 16:20 | DWR | Iona Branscum | 4060 | 000039 |
| Smith X Lease, Equinox Oil Co. | Oil brine | 23S-23W-1B (N/2) | 3/9/00 | KCC | 000040 | |||
| Oliphant #1, Palomino Pet. | Oil brine | 23S-23W-11AA (Center) | 3/9/00 | KCC | 000041 |
Figure 7--Location of April 9, 2000, water sampling sites (underlined). A larger version of this figure is available as an Acrobat PDF file.
Table 5 lists the chemical data for the water samples analyzed by the KGS laboratory. The water and brine samples were filtered through 0.45 μm membrane filter paper before analysis. Specific conductance was measured to estimate chloride concentrations and determine dilution factors for the optimum concentration range of the analytical method. Chloride, sulfate, and bromide concentrations were determined using automated colorimetric methods, except for sulfate content in the brine samples, which was measured by a turbidimetric procedure. The estimated maximum errors in the chloride, sulfate, and bromide determinations are 3%, 5%, and 5%, respectively, except for the bromide in the samples with concentrations <0.2 mg/L, for which the error is estimated as ±0.01 mg/L. The error estimates are based on results from participation in the standard reference water program of the U.S. Geological Survey and on data previously obtained for samples spiked with standards. The charge-balance errors for all of the samples are <3%; the errors for the ground waters are all <1%.
Table 5--Chemical data for water samples collected as part of the KGS-DWR study of the Smith/Wasko study area in 2000.
| Sample site | KGS lab number |
Lab Sp.C. μS/cm |
pH units |
Ca mg/L |
Mg mg/L |
Na mg/L |
K mg/L |
Sr mg/L |
CO3 mg/L |
HCO3 mg/L |
Cl mg/L |
SO4 mg/L |
NO3-N mg/L |
Br mg/L |
TDS sum mg/L |
C.B.E.a % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW-1 | 000030 | 3280 | 7.35 | 398 | 54.5 | 326 | 21.4 | 2.56 | 470 | 298 | 1090 | 12.3 | 0.81 | 2480 | -0.7 | |
| MW-2 | 000031 | 2230 | 7.40 | 223 | 49.1 | 194 | 12.3 | 2.12 | 264 | 238 | 524 | 32.5 | 0.63 | 1517 | -0.7 | |
| MW-2, Sample 2 | 000032 | 2400 | 7.45 | 256 | 60.4 | 193 | 13.9 | 2.44 | 263 | 253 | 576 | 42.9 | 0.70 | 1674 | -0.1 | |
| MW-16 | 000033 | 4080 | 7.20 | 458 | 85.9 | 432 | 23.7 | 3.66 | 374 | 444 | 1370 | 23.7 | 0.97 | 3100 | 0.5 | |
| MW-17 | 000034 | 4280 | 7.25 | 502 | 84.0 | 445 | 26.4 | 3.61 | 431 | 482 | 1440 | 18.0 | 1.14 | 3270 | 0.2 | |
| MW-18 | 000035 | 3520 | 7.20 | 414 | 80.0 | 314 | 16.8 | 3.36 | 362 | 490 | 924 | 23.5 | 1.08 | 2520 | 0.8 | |
| SE Windmill | 000036 | 1350 | 7.40 | 186 | 22.1 | 68.1 | 15.3 | 1.01 | 435 | 147 | 144 | 0.2 | 0.40 | 799 | 0.6 | |
| Rock Creek 1 | 000037 | 718 | 8.00 | 86.8 | 16.3 | 29.2 | 7.0 | 0.65 | 175 | 85.9 | 82.2 | 0.2 | 0.12 | 395 | 0.8 | |
| Rock Creek 2 | 000038 | 1175 | 8.00 | 151 | 24.7 | 57.1 | 12.3 | 1.00 | 329 | 135 | 131 | 1.4 | 0.15 | 681 | 1.4 | |
| Rock Creek 3 | 000039 | 1000 | 8.45 | 113 | 22.4 | 54.4 | 13.9 | 0.86 | 6.4 | 208 | 131 | 124 | 0.2 | 0.08 | 563 | 2.61 |
| Smith X Lease brine | 000040 | 72000 | NDb | 1762 | 488 | 16360 | 330 | 52.1 | NDb | 26490 | 2670 | 46.2 | 48500 | 2.21 | ||
| Oliphant #1 brine | 000041 | 73300 | 7.40 | 1572 | 549 | 16600 | 342 | 53.1 | 537 | 28330 | 1470 | 56.6 | 49300 | 0.9 | ||
| a Charge balance error b Not determined because the sample contained substantial H2S that could potentially interfere with the pH electrode used in the alkalinity titration. |
||||||||||||||||
The chemical data in Tables 2, 3, and 5 indicate that the saline ground waters (total dissolved solids [TDS] concentration >1,000 mg/L) in the alluvial aquifer of the Smith/Wasko site are high in both chloride and sulfate concentrations. The waters are saline and calcium, sodium-sulfate, chloride in chemical type. However, the ground water from the windmill site (Table 5) is fresh (TDS <1,000 mg/L) and contains chloride and sulfate contents <150 mg/L. The waters collected from Rock Creek were also fresh with chloride and sulfate concentrations all <140 mg/L at time of collection. The sample from the Jetmore water supply was fresh and contained chloride and sulfate contents each less than 120 mg/L.
Except for sample TH-3, the sulfate concentration was greater than that of chloride for all the saline ground-water samples with charge-balance errors close to or less than 15%. The chloride and sulfate concentration ranges were 126-786 mg/L and 236-1,440 mg/L for the saline ground-water analyses with errors >10%. The sulfate and chloride contents were relatively similar for the fresh ground and surface waters. The ranges for calcium, magnesium, and sodium concentrations were 190-525 mg/L, 29-99 mg/L, and 99-465 mg/L, respectively, in the saline ground-water samples with errors >10%. Nitrate-nitrogen contents in the ground waters ranged widely from low values of <1 mg/L to very high values of >40 mg/L. Many of the saline ground waters contained more nitrate-nitrogen than the maximum contaminant level allowed for public drinking water supplies.
The oil brines collected from the study area had chloride concentrations within the range 26,000-29,000 mg/L chloride. The sulfate concentrations in the oil brines were higher than for many saltwaters produced with oil in Kansas but were still much lower than the chloride contents.
Ratios of selected chemical constituents (Table 6) were examined to determine the salinity sources affecting the saline ground waters in the alluvial aquifer. The ratios of the saline ground waters were assessed relative to the different ratios of potential source waters with a consideration for how the ratios would be expected to change during and transport and mixing of the saline sources with freshwater in the area. In addition, natural processes and anthropogenic activities that can affect the chemistry of ground waters were considered. One ratio in Table 6 that particularly stands out as being substantially different for the saline ground waters from the monitoring wells compared to the freshwaters and oil brines is the high sulfate/chloride ratio.
Table 6--Chemical ratios for water samples collected as part of the KGS-DWR study of the Smith/Wasko study area in 2000.
| Sample site | KGS lab number |
Na/Cl mass ratio |
Ca/Mg mass ratio |
Ca/SO4 mass ratio |
SO4/Cl mass ratio |
(Ca+Mg)/Na equivalent ratio |
Br/Cl mass ratio x10,000 |
NO3/Cl mass ratio |
|---|---|---|---|---|---|---|---|---|
| MW-1 | 000030 | 1.095 | 7.30 | 0.365 | 3.658 | 1.717 | 27.3 | 0.0412 |
| MW-2 | 000031 | 0.816 | 4.54 | 0.425 | 2.202 | 1.793 | 26.5 | 0.1367 |
| MW-2, Sample 2 | 000032 | 0.762 | 4.23 | 0.444 | 2.277 | 2.115 | 27.8 | 0.1696 |
| MW-16 | 000033 | 0.974 | 5.33 | 0.335 | 3.081 | 1.590 | 21.9 | 0.0534 |
| MW-17 | 000034 | 0.924 | 5.98 | 0.350 | 2.977 | 1.650 | 23.7 | 0.0374 |
| MW-18 | 000035 | 0.640 | 5.17 | 0.448 | 1.886 | 1.997 | 22.1 | 0.0479 |
| SE Windmill | 000036 | 0.463 | 8.41 | 1.293 | 0.980 | 3.749 | 27.4 | 0.0011 |
| Rock Creek 1 | 000037 | 0.340 | 5.32 | 1.056 | 0.957 | 4.471 | 14.1 | 0.0018 |
| Rock Creek 2 | 000038 | 0.423 | 6.11 | 1.154 | 0.970 | 3.855 | 11.0 | 0.0107 |
| Rock Creek 3 | 000039 | 0.415 | 5.04 | 0.912 | 0.947 | 3.165 | 6.1 | 0.0014 |
| Smith X Lease brine | 000040 | 0.617 | 3.61 | 0.660 | 0.101 | 0.180 | 17.4 | - |
| Oliphant #1 brine | 000041 | 0.585 | 2.86 | 1.069 | 0.0519 | 0.171 | 20.0 | - |
Procedures for the geochemical identification methods used are described in Whittemore (1984 and 1995). These methods primarily include plots of the constituent mass ratios bromide/chloride and sulfate/chloride versus chloride concentration, and points for the water sample data and curves for the mixing of different source waters. Each mixing curve is generated using an algebraic equation for conservative mixing of two end-member waters. Conservative mixing refers to the simple mixing of waters without chemical reactions, such as mineral precipitation or adsorption, which could alter the concentrations of one or both of the constituents. The intersection of two mixing curves can be determined graphically or by solving for simultaneous algebraic equations. The bromide/chloride ratio is multiplied by 10,000 for graphical display to give numbers that range from about one upwards (the lowest mass ratios are near 0.0001 for halite or rock salt dissolution). The logarithmic scales are used in the graphs because they produce a more even distribution (separation) of points for large ranges in concentration and ratios than linear scales.
Figure 8 is a bromide/chloride versus chloride concentration plot containing points for ground and surface waters and oil brines from the study area, and ground waters from the Dakota aquifer in Hodgeman County. The ground waters from the Dakota aquifer with less than 200 mg/L chloride were sampled from domestic, stock, and irrigation wells completed in the Dakota Formation and analyzed by the KGS as a part of a previous study of the Dakota aquifer in Kansas (see KGS Internet site for Dakota Aquifer Program http://www.kgs.ku.edu/Dakota/vol1/index.html). The saltwater with a chloride content of about 10,000 mg/L was collected from an observation well screened in the Cheyenne Sandstone part of the Dakota aquifer a few miles south of Jetmore. The two solid curves in Figure 8 enclose a zone in which points would occur if freshwaters in Hodgeman County mixed with the saltwater from the Cheyenne Sandstone. The bromide/chloride ratios of saltwaters in the Dakota aquifer are relatively low because the source is primarily from upward intrusion of saltwater from underlying Permian formations. The saltwaters in the Permian rocks underlying the Dakota aquifer in central and western Kansas are generally derived from dissolution of rock salt, which has a low bromide/chloride ratio. The dashed curve in Figure 8 represents the location of where points would fall for mixtures of freshwater (in particular, the ground water from the windmill well) with the oil-field brines from the study area. Formation brines associated with hydrocarbons in strata not containing rock salt in Kansas have substantially higher bromide/chloride ratios than for saltwater primarily derived from the dissolution of rock salt.
Figure 8--Bromide/chloride mass ratio versus chloride concentration for ground and surface waters and oil brines from the Smith/Wasko area, and ground waters from the Dakota aquifer in Hodgeman County. Symbol representation: 1 and 2 = monitoring wells 1 and 2; 6, 7, 8 = monitoring wells 16, 17, 18; R = Rock Creek; D = Dakota aquifer; O = oil brine.
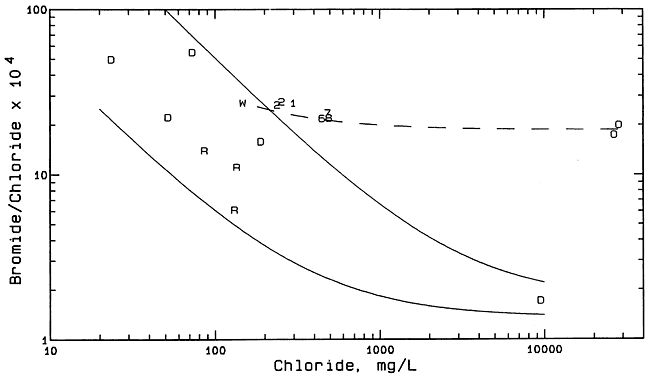
The locations of the points for the Rock Creek samples within the zone of mixing between freshwater and saltwater from the Dakota aquifer suggest that the main source of chloride in the creek samples could be from the Dakota aquifer. The locations of the points for the saline water samples collected from the monitoring wells in the Smith/Wasko site do not fit the simple mixing of freshwaters with only a saltwater source from the Dakota aquifer because the points lie above the zone for this type of mixture. The points appear to be consistent with the mixing of freshwater from the Dakota aquifer with oil brine. However, the sulfate/chloride ratios for the saline ground waters in the alluvium of the Smith/Wasko site are not consistent with oil brine as a source.
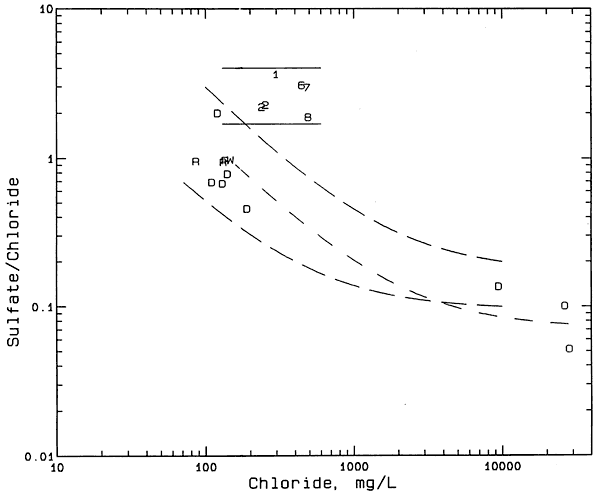
Figure 9 is a sulfate/chloride versus chloride concentration plot containing points for ground and surface waters and oil brines from the study area, ground waters from the Dakota aquifer in T. 23 S., R. 23 W., and the Cheyenne Sandstone a few miles south of Jetmore. The ground waters from the Dakota aquifer within the township in which the Smith/Wasko site is located were collected and analyzed by the U.S. Geological Survey in 1973. The two curves with the longer dashes in Figure 9 comprise a zone of mixing of freshwaters from the Dakota aquifer with the saltwater from the Cheyenne Sandstone. The curve with the shorter dashes represents where points would be located for the mixing of water from the windmill well at the Smith/Wasko site with the average oil-brine in the study area. The sulfate/chloride ratios for the saline ground waters in the alluvial aquifer at the site are substantially greater than would be expected for mixing with either the saltwater deep in the Dakota aquifer or oil brine from the area. Based on the sulfate/chloride mixing plot (Figure 9), the primary source of the chloride in the saline ground waters of the Smith/Wasko site could not come from either oil brine or mixing with the saltwater from deeper in the Dakota aquifer.
Figure 9--Sulfate/chloride mass ratio versus chloride concentration for ground and surface waters and oil brines from the Smith/Wasko area, and ground waters from the Dakota aquifer in the study area. Symbol representation: 1 and 2 = monitoring wells 1 and 2; 6, 7, 8 = monitoring wells 16, 17, 18; R = Rock Creek; D = Dakota aquifer; O = oil brine.
A process that has increased the TDS content of both surface and ground waters in the Great Plains is concentration of dissolved salts by evapotranspiration. Consumption of water by evaporation and plant transpiration reduces the original volume of the water in which the salts were dissolved, thereby increasing the salt concentration in the residual solution. All constituents dissolved in the water are increased in concentration until the solubility of selected minerals is exceeded and the minerals precipitate as solids, or the increased TDS affects the relative amounts of dissolved constituents that can be adsorbed on the surfaces of solids in soils and sediments. In general, the ratio of bromide/chloride in water does not change appreciably during the evapotranspiration process. The sulfate/chloride ratio does not usually change appreciably until the solubility of gypsum is exceeded.
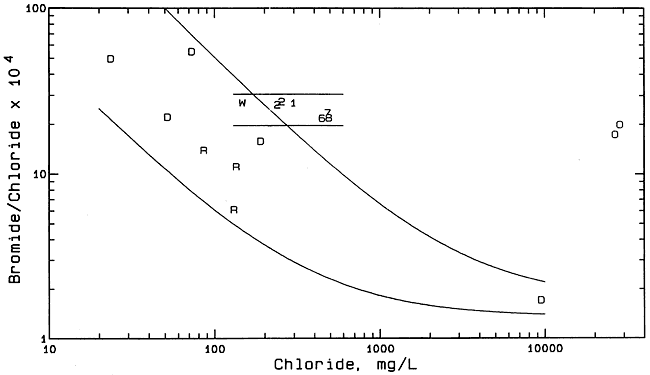
The two solid lines in Figure 9 enclose the zone in which points would be located if the process of evapotranspiration concentration was primarily responsible for generating the salinity in the ground waters in the alluvial aquifer of the Smith/Wasko site. The saline ground waters at the Smith/Wasko site contain dissolved calcium and sulfate contents that do not exceed the solubility of gypsum. Extension of the two solid lines to lower chloride contents indicates that at least one Dakota ground water from the area would fit in the zone. Figure 10 is a plot of bromide/chloride versus chloride content that includes the same sample points and the same solid curves for the mixing of freshwater with saltwater in the Dakota aquifer in the study region as in Figure S. However, Figure 10 includes two solid, horizontal lines that enclose the zone in which points would be located if the process of evapotranspiration concentration generated the salinity in the alluvial ground waters. The salinity source identification must be consistent with changes in both the bromide/chloride and sulfate/chloride ratios. Thus, Figures 9 and 10 are consistent with the evapotranspiration concentration process as the main origin of the salinity in the alluvial ground waters.
Figure 10--Bromide/chloride mass ratio versus chloride concentration for ground and surface waters and oil brines from the Smith/Wasko area, and ground waters from the Dakota aquifer in Hodgeman County. Symbol representation: 1 and 2 = monitoring wells 1 and 2; 6, 7, 8 = monitoring wells 16, 17, 18; R = Rock Creek; D = Dakota aquifer; O = oil brine.
The nitrate concentrations (and nitrate/chloride ratios) are all substantially larger in the saline waters from the monitoring wells in the alluvial aquifer than in freshwaters and saltwaters in the Dakota aquifer and in oil brines. The interpretation of the chemistry of the saline alluvial waters must also account for the nitrate distribution. Based on the data for the waters from the study and surrounding areas, the high nitrate concentrations and nitrate/chloride ratios could not be derived from natural waters in the Dakota aquifer or flow from Rock Creek entering the valley; another source is needed. The most probable source is nitrate derived from nitrogen-containing fertilizer associated with the irrigated agriculture. Nitrate from this source could elevate the concentration of dissolved nitrate in the soil water that had become saline by the evapotranspiration concentration process. Saline water with high nitrate content that infiltrated below the root zone of the crops and reached the underlying alluvial aquifer would then increase not only the salinity of the ground water in the aquifer but also the nitrate content. Therefore, the high nitrate concentrations also support the source of salinity as derived from the irrigation return recharge that has been concentrated by evapotranspiration as the primary cause of the salinity.
The primary source of salinity fits the concentration of natural sources of dissolved salts in the ground waters of the alluvial aquifer by the process of evapotranspiration mainly associated with irrigation in the Buckner Creek valley. The natural source of the sulfate is probably dissolution of gypsum in Cretaceous shale underlying part of the alluvial aquifer. The natural source of most of the chloride is probably ground water in the Dakota aquifer also underlying part of the alluvial valley. Assuming 78-80 percent consumption of the alluvial ground water used during irrigation, the original dissolved solids in the water would be concentrated by a factor of 4-5 during an irrigation season. The saline return flow from the irrigation can recharge the alluvial aquifer and thereby increase the ground-water salinity. High nitrate concentrations in the alluvial aquifer water in comparison with low values in the Dakota aquifer also fit the return recharge as the primary cause of the salinity.
Higher ground-water salinities appear to be associated with years of low precipitation and streamflow. During some summers, the streamflow is zero or near zero. Pumping from the alluvial aquifer would be expected to reduce ground-water discharge to the stream and exacerbate low streamflow conditions. Small or zero baseflow to the stream means that saline ground water is not flushed from the alluvial aquifer but accumulates in the valley. Periods of greater precipitation would be expected to result in less irrigation water use, greater recharge to the alluvial aquifer, and flushing of some of the salinity buildup through discharge to the stream. Thus, the salinity of the alluvial ground waters could decrease following extended wet periods and increase during long dry periods.
TEST, 1993, Groundwater site investigation report, Smith/Wasko brine contamination site, Hodgeman County, Kansas: Submitted to Kansas Department of Health and Environment by Total Environmental Services and Technologies, Shawnee, KS, January 29, 1993.
Macfarlane, P.A., Whittemore, D.O., Schloss, J.A., Jayatilake, H., Yoder, S., Mitchell, J.H., and McClain, T.J., 1996, Geographic information system coverages of the Kansas regional aquifer systems: Kansas Geological Survey Open-File Report 96-1b, 10 p. (Digital data for the Dakota aquifer extent is available through the Internet site of the Data Access and Support Center, http://www.kansasgis.org/)
Macfarlane, P.A., Doveton, J.H., and Whittemore, D.O., 1998, User's guide to the Dakota aquifer in Kansas: Kansas Geological Survey, Technical Series 2, 56 p. [available online]
Whittemore, D.O., 1984, Geochemical identification of salinity sources; in, R.H. French (ed.), Salinity in Watercourses and Reservoirs (Proceedings of the International Conference on State-of-the-Art Control of Salinity): Ann Arbor Science, Butterworth Publishers, Stoneham, MA, p. 505-514.
Whittemore, D.O., 1995, Geochemical differentiation of oil and gas brine from other saltwater sources contaminating water resources: Case studies from Kansas and Oklahoma: Environmental Geosciences, vol. 2, no. 1, 15-31.
Zeller, D.E., 1968, The stratigraphic succession in Kansas: Kansas Geological Survey Bulletin 189, 81 p. [available online]
Kansas Geological Survey, Geohydrology
Placed online Sept. 14, 2007, original report dated 2001
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Hydro/Publications/2001/OFR01_61/index.html